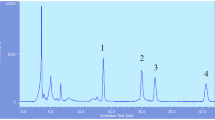
Abstract
Long-chain fatty acids (LCFA) are commonly found in lipid-rich wastewaters and are a key factor to monitor the anaerobic digesters. A new simple, fast, precise, and suitable method for routine analysis of LCFA is proposed. The system involves in-syringe-magnetic stirring-assisted dispersive liquid-liquid microextraction (DLLME) prior to gas chromatography-mass spectrometry (GC-MS) without a derivatization process. Calibration curves were prepared in an ethanol solution (R2 ≥ 0.996), which was also useful as disperser solvent. Hexane was chosen as the extraction solvent. Several parameters (pH, ionic strength, extraction solvent volume, stirring time) were optimized in multivariate and univariate studies. Limits of detection (LODs) were found in the range 0.01–0.05 mg L−1 and good precision inter-day (RSDs≤7.9%) and intra-day (RSDs≤4.4%) were obtained. The method was applied to quantify LCFA in supernatants of anaerobic digesters and olive mill wastewaters (OMW). Palmitic, stearic, and oleic acids were the most abundant fatty acid in the analyzed samples and the relative recoveries for all of them were between 81 and 113%.
Graphical abstract





Similar content being viewed by others
References
Stoll U, Gupta H. Management strategies for oil and grease residues. Waste Manag Res. 1997;15:23–32.
Kabouris JC, Tezel U, Pavlostathis SG, Engelmann M, Dulaney JA, Todd AC, et al. Mesophilic and thermophilic anaerobic digestion of municipal sludge and fat, oil, and grease. Water Environ Res. 2009;81:476–85.
Chipasa KB, Mȩdrzycka K. Behavior of lipids in biological wastewater treatment processes. J Ind Microbiol Biotechnol. 2006;33:635–45.
Chowdhury B, Lin L, Dhar BR, Islam MN, McCartney D, Kumar A. Enhanced biomethane recovery from fat, oil, and grease through co-digestion with food waste and addition of conductive materials. Chemosphere. 2019;236:124362.
Singh S, Rinta-Kanto JM, Kettunen R, Tolvanen H, Lens P, Collins G, et al. Anaerobic treatment of LCFA-containing synthetic dairy wastewater at 20 °C: process performance and microbial community dynamics. Sci Total Environ. 2019;691:960–8.
Farrington JW, Quinn JG. Petroleum hydrocarbons and fatty acids in wastewater effluents. J Water Pollut Control Fed. 1973;45:704–12.
Cadavid-Rodríguez LS, Vargas-Muñoz MA, Plácido J. Biomethane from fish waste as a source of renewable energy for artisanal fishing communities. Sustain Energy Technol Assessments. 2019;34:110–5.
Ning Z, Zhang H, Li W, Zhang R, Liu G, Chen C. Anaerobic digestion of lipid-rich swine slaughterhouse waste: methane production performance, long-chain fatty acids profile and predominant microorganisms. Bioresour Technol. 2018;269:426–33.
Kobayashi T, Kuramochi H, Maeda K, Xu K. A simple method for the detection of long-chain fatty acids in an anaerobic digestate using a quartz crystal sensor. Energies. 2017;10:1–14.
Khdair AI, Abu-Rumman G, Khdair SI. Pollution estimation from olive mills wastewater in Jordan. Heliyon. 2019;5:e02386.
Angelidaki I, Ahring BK. Effects of free long-chain fatty acids on thermophilic anaerobic digestion. Appl Microbiol Biotechnol. 1992;37:808–12.
Chen JL, Ortiz R, Steele TWJ, Stuckey DC. Toxicants inhibiting anaerobic digestion: a review. Biotechnol Adv. 2014;32:1523–34.
Lalman JA, Bagley DM. Extracting long-chain fatty acids from a fermentation medium. JAOCS, J Am Oil Chem Soc. 2004;81:105–10.
Siang GH, Makahleh A, Saad B, Lim BP. Hollow fiber liquid-phase microextraction coupled with gas chromatography-flame ionization detection for the profiling of fatty acids in vegetable oils. J Chromatogr A. 2010;1217:8073–8.
Zhang H, Wang Z, Liu O. Development and validation of a GC-FID method for quantitative analysis of oleic acid and related fatty acids. J Pharm Anal. 2015;5:223–30.
Aparicio-Ruiz R, García-González DL, Morales MT, Lobo-Prieto A, Romero I. Comparison of two analytical methods validated for the determination of volatile compounds in virgin olive oil: GC-FID vs GC-MS. Talanta. 2018;187:133–41.
Vičanová J, Tvrzická E, Štulík K. Capillary gas chromatography of underivatized fatty acids with a free fatty acid phase column and a programmed temperature vaporizer injector. J Chromatogr B. 1994;656:45–50.
Kaluzny MA, Duncan LA, Merritt MV, Epps DE. Rapid separation of lipid classes in high yield and purity using bonded phase columns. J Lipid Res. 1985;26:135–40.
Jiang Y, Zhang Y, Banks CJ. Determination of long chain fatty acids in anaerobic digesters using a rapid non-derivatisation GC-FID method. Water Sci Technol. 2012;66:741–7.
Añorve-Morga J, Castañeda-Ovando A, Cepeda-Saez A, Archibold AD, Jaimez-Ordaz J, Contreras-López E, et al. Microextraction method of medium and long chain fatty acids from milk. Food Chem. 2015;172:456–61.
Meng Z, Wen D, Sun D, Gao F, Li W, Liao Y, et al. Rapid determination of C12-C26 non-derivatized fatty acids in human serum by fast gas chromatography. J Sep Sci. 2007;30:1537–43.
Bligh EG, Dyer WJ. Rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–7.
Procida G, Ceccon L. Gas chromatographic determination of free fatty acids in olive mill waste waters. Anal Chim Acta. 2006;561:103–6.
Neves L, Pereira MA, Mota M, Alves MM. Detection and quantification of long chain fatty acids in liquid and solid samples and its relevance to understand anaerobic digestion of lipids. Bioresour Technol. 2009;100:91–6.
Rezaee M, Yamini Y, Faraji M. Evolution of dispersive liquid-liquid microextraction method. J Chromatogr A. 2010;1217:2342–57.
Quigley A, Connolly D, Cummins W. The application of dispersive liquid–liquid microextraction in the analyses of the fatty acid profile in bovine milk in response to changes in body condition score. J Chromatogr B. 2018;1073:130–5.
Pusvaskiene E, Januskevic B, Prichodko A, Vickackaite V. Simultaneous derivatization and dispersive liquid-liquid microextraction for fatty acid GC determination in water. Chromatographia. 2009;69:271–6.
Makoś P, Fernandes A, Boczkaj G. Method for the determination of carboxylic acids in industrial effluents using dispersive liquid-liquid microextraction with injection port derivatization gas chromatography–mass spectrometry. J Chromatogr A. 2017;1517:26–34.
Horstkotte B, Suárez R, Solich P, Cerdà V. In-syringe-stirring: a novel approach for magnetic stirring-assisted dispersive liquid-liquid microextraction. Anal Chim Acta. 2013;788:52–60.
Maya F, Horstkotte B, Estela JM, Cerdà V. Lab in a syringe: fully automated dispersive liquid-liquid microextraction with integrated spectrophotometric detection. Anal Bioanal Chem. 2012;404:909–17.
Clavijo S, Avivar J, Suárez R, Cerdà V. In-syringe magnetic stirring-assisted dispersive liquid-liquid microextraction and silylation prior gas chromatography-mass spectrometry for ultraviolet filters determination in environmental water samples. J Chromatogr A. 2016;1443:26–34.
Alexovič M, Horstkotte B, Šrámková I, Solich P, Sabo J. Automation of dispersive liquid–liquid microextraction and related techniques. Approaches based on flow, batch, flow-batch and in-syringe modes. TrAC - Trends Anal Chem. 2017;86:39–55.
Pruksatrakul T, Phoopraintra P, Wilairat P, Chaiyen P, Chantiwas R. Development of a sequential injection-liquid microextraction procedure with GC-FID for analysis of short-chain fatty acids in palm oil mill effluent. Talanta. 2017;165:612–8.
International Union of Pure and Applied Chemistry IUPAC. Harmonized guidelines for single-laboratory validation of methods of analysis.
Barwick VJ. Strategies for solvent selection - a literature review. TrAC - Trends Anal Chem. 1997;16:293–309.
Mansour EA, Abo El-Enin SA, Hamouda AS, Mahmoud HM. Efficacy of extraction techniques and solvent polarity on lipid recovery from domestic wastewater microalgae. Environ Nanotechnology, Monit Manag. 2019;12:100271.
Cistola DP, Hamilton JA, Jackson D, Small DM. Ionization and phase behavior of fatty acids in water: application of the Gibbs phase rule. Biochemistry. 1988;27:1881–8.
Rahman MA, Ghosh AK, Bose RN. Dissociation constants of long chain fatty acids in methanol-water and ethanol-water mixtures. J Chem Technol Biotechnol. 2007;29:158–62.
Kanicky JR, Shah DO. Effect of degree, type, and position of unsaturation on the pKa of long-chain fatty acids. J Colloid Interface Sci. 2002;256:201–7.
Pan L, Adams M, Pawliszyn J. Determination of fatty acids using solid phase microextraction. Anal Chem. 1995;67:4396–403.
González Casado A, Alonso Hernández EJ, Vílchez JL. Determination of fatty acids (C8-C22) in urban wastewater by GC-MS. Water Res. 1998;32:3168–72.
Wu J, Lee HK. Ion-pair dynamic liquid-phase microextraction combined with injection-port derivatization for the determination of long-chain fatty acids in water samples. J Chromatogr A. 2006;1133:13–20.
National Center for Biotechnology Information (2020). PubChem compound summary for CID 23665730, Sodium oleate. https://pubchem.ncbi.nlm.nih.gov/compound/Sodium-oleate
Chan PC, de Toledo RA, Shim H. Anaerobic co-digestion of food waste and domestic wastewater – effect of intermittent feeding on short and long chain fatty acids accumulation. Renew Energy. 2018;124:129–35.
Alves MM, Pereira MA, Sousa DZ, Cavaleiro AJ, Picavet M, Smidt H, et al. Waste lipids to energy: how to optimize methane production from long-chain fatty acids (LCFA). Microb Biotechnol. 2009;2:538–50.
Marie Wong, Laurence Eyres LR. Green vegetable oil processing: 2- modern aqueous oil extraction—centrifugation systems for olive and avocado oils, First ed. Elsevier.
Acknowledgements
Thanks to Emaya and La Almazara de Can Det (olive oil company) for the provision of wastewater samples.
Funding
The authors received financial support from the Comunitat Autonoma de les Illes Balears through the Direcció General de Política Universitaria i Recerca with funds from the Tourist Stay Tax Law (PRD2018/45). M.A.V-M. received the support from the Spanish Ministry of Science, Innovation and Universities (MCIU) for the pre-doctoral research fellowship (FPU19/06082).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOC 717 kb)
Rights and permissions
About this article
Cite this article
Vargas-Muñoz, M.A., Cerdà, V., Turnes Palomino, G. et al. Determination of long-chain fatty acids in anaerobic digester supernatant and olive mill wastewater exploiting an in-syringe dispersive liquid-liquid microextraction and derivatization-free GC-MS method. Anal Bioanal Chem 413, 3833–3845 (2021). https://doi.org/10.1007/s00216-021-03338-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-021-03338-z