Abstract
In this report, we have designed a rapid and sensitive, intensity-based ratiometric sensing as well as lifetime-based sensing probe for the detection of hyaluronidase activity. Hyaluronidase expression is known to be upregulated in various pathological conditions. We have developed a fluorescent probe by heavy labeling of hyaluronic acid with a new orange/red-emitting organic azadioxatriangulenium (ADOTA) fluorophore, which exhibits a long fluorescence lifetime (∼20 ns). The ADOTA fluorophore in water has a peak fluorescence lifetime of ∼20 ns and emission spectra centered at 560 nm. The heavily ADOTA-labeled hyaluronic acid (HA-ADOTA) shows a red shift in the peak emission wavelength (605 nm), a weak fluorescence signal, and a shorter fluorescence lifetime (∼4 ns) due to efficient self-quenching and formation of aggregates. In the presence of hyaluronidase, the brightness and fluorescence lifetime of the sample increase with a blue shift in the peak emission to its original wavelength at 560 nm. The ratio of the fluorescence intensity of the HA-ADOTA probe at 560 and 605 nm can be used as the sensing method for the detection of hyaluronidase. The cleavage of the hyaluronic acid macromolecule reduces the energy migration between ADOTA molecules, as well as the degree of self-quenching and aggregation. This probe can be efficiently used for both intensity-based ratiometric sensing as well as fluorescence lifetime-based sensing of hyaluronidase. The proposed method makes it a rapid and sensitive assay, useful for analyzing levels of hyaluronidase in relevant clinical samples like urine or plasma.

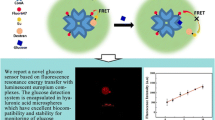
Scheme showing cleavage of HA-ADOTA probe by hyaluronidase and the change in the emission spectrum of HA-ADOTA probe before and after cleavage by hyaluronidase










Similar content being viewed by others
References
Stern R. Hyaluronidases in cancer biology. Semin Cancer Biol. 2008;18(4):275–80.
Chao KL, Muthukumar L, Herzberg O. Structure of human hyaluronidase-1, a hyaluronan hydrolyzing enzyme involved in tumor growth and angiogenesis. Biochemistry (N Y). 2007;46(23):6911–20.
Bollet AJ, Bonner WM, Nance JL. The presence of hyaluronidase in various mammalian tissues. J Biol Chem. 1963;238:3522–7.
Franzmann EJ, Schroeder GL, Goodwin WJ, Weed DT, Fisher P, Lokeshwar VB. Expression of tumor markers hyaluronic acid and hyaluronidase (HYAL1) in head and neck tumors. Int J Cancer. 2003;106(3):438–45.
Liu D, Pearlman E, Diaconu E, Guo K, Mori H, Haqqi T, et al. Expression of hyaluronidase by tumor cells induces angiogenesis in vivo. Proc Natl Acad Sci U S A. 1996;93(15):7832–7.
Lokeshwar VB, Estrella V, Lopez L, Kramer M, Gomez P, Soloway MS, et al. HYAL1-v1, an alternatively spliced variant of HYAL1 hyaluronidase: a negative regulator of bladder cancer. Cancer Res. 2006;66(23):11219–27.
Chain E, Duthie E. Identity of hyaluronidase and spreading factor. Br J Exp Pathol. 1940;21(6):324.
Hobby GL, Dawson MH, Meyer K, Chaffee E. The relationship between spreading factor and hyaluronidase. J Exp Med. 1941;73(1):109–23.
McCUTCHEON M, COMAN DR. Spreading factor in human carcinomas. Cancer Res. 1947;7(6):379–82.
Turley EA, Noble PW, Bourguignon LY. Signaling properties of hyaluronan receptors. J Biol Chem. 2002;277(7):4589–92.
Girish K, Kemparaju K. The magic glue hyaluronan and its eraser hyaluronidase: a biological overview. Life Sci. 2007;80(21):1921–43.
Stern R. Hyaluronan catabolism: a new metabolic pathway. Eur J Cell Biol. 2004;83(7):317–25.
Stern R. Hyaluronan metabolism: a major paradox in cancer biology. Pathol Biol. 2005;53(7):372–82.
Stern R, Asari AA, Sugahara KN. Hyaluronan fragments: an information-rich system. Eur J Cell Biol. 2006;85(8):699–715.
Lokeshwar VB, Rubinowicz D, Schroeder GL, Forgacs E, Minna JD, Block NL, et al. Stromal and epithelial expression of tumor markers hyaluronic acid and HYAL1 hyaluronidase in prostate cancer. J Biol Chem. 2001;276(15):11922–32.
DORFMAN A, OTT ML. A turbidimetric method for the assay of hyaluronidase. J Biol Chem. 1948;172(2):367–75.
Knudsen P, Koefoed J. Viscometric determination of hyaluronidase activity in biological fluids. Scand J Clin Lab Invest. 1961;13(4):673–82.
Stern M, Stern R. An ELISA-like assay for hyaluronidase and hyaluronidase inhibitors. Matrix. 1992;12(5):397–403.
Pham HT, Block NL, Lokeshwar VB. Tumor-derived hyaluronidase: a diagnostic urine marker for high-grade bladder cancer. Cancer Res. 1997;57(4):778–83.
Bonner W, Cantey EY. Colorimetric method for determination of serum hyaluronidase activity. Clin Chim Acta. 1966;13(6):746–52.
Steiner B, Cruce D. A zymographic assay for detection of hyaluronidase activity on polyacrylamide gels and its application to enzymatic activity found in bacteria. Anal Biochem. 1992;200(2):405–10.
Podyma KA, Yamagata S, Sakata K, Yamagata T. Difference of hyaluronidase produced by human tumor cell lines with hyaluronidase present in human serum as revealed by zymography. Biochem Biophys Res Commun. 1997;241(2):446–52.
Cheng D, Han W, Yang K, Song Y, Jiang M, Song E. One-step facile synthesis of hyaluronic acid functionalized fluorescent gold nanoprobes sensitive to hyaluronidase in urine specimen from bladder cancer patients. Talanta. 2014;130:408–14.
Chib R, Raut S, Fudala R, Chang A, Mummert M, Rich R, et al. FRET based ratio-metric sensing of hyaluronidase in synthetic urine as a biomarker for bladder and prostate cancer. Curr Pharm Biotechnol. 2013;14(4):470–4.
Fudala R, Mummert ME, Gryczynski Z, Rich R, Borejdo J, Gryczynski I. Lifetime-based sensing of the hyaluronidase using fluorescein labeled hyaluronic acid. J Photochem Photobiol B Biol. 2012;106:69–73.
Huang Y, Song C, Li H, Zhang R, Jiang R, Liu X, et al. Cationic conjugated polymer/hyaluronan-doxorubicin complex for sensitive fluorescence detection of hyaluronidase and tumor-targeting drug delivery and imaging. ACS Appl Mater Interfaces. 2015;7(38):21529–37.
Liu S, Zhao N, Cheng Z, Liu H. Amino-functionalized green fluorescent carbon dots as surface energy transfer biosensors for hyaluronidase. Nanoscale. 2015;7(15):6836–42.
Murai T, Kawashima H. A simple assay for hyaluronidase activity using fluorescence polarization. Biochem Biophys Res Commun. 2008;376(3):620–4.
Wang Z, Li X, Song Y, Li L, Shi W, Ma H. An upconversion luminescence nanoprobe for the ultrasensitive detection of hyaluronidase. Anal Chem. 2015;87(11):5816–23.
Rich RM, Mummert M, Foldes-Papp Z, Gryczynski Z, Borejdo J, Gryczynski I, et al. Detection of hyaluronidase activity using fluorescein labeled hyaluronic acid and fluorescence correlation spectroscopy. J Photochem Photobiol B Biol. 2012;116:7–12.
Fudala R, Mummert ME, Gryczynski Z, Gryczynski I. Fluorescence detection of hyaluronidase. J Photochem Photobiol B Biol. 2011;104(3):473–7.
Hu Q, Zeng F, Wu S. A ratiometric fluorescent probe for hyaluronidase detection via hyaluronan-induced formation of red-light emitting excimers. Biosens Bioelectron. 2016;79:776–83.
Song Y, Wang Z, Li L, Shi W, Li X, Ma H. Gold nanoparticles functionalized with cresyl violet and porphyrin via hyaluronic acid for targeted cell imaging and phototherapy. Chem Commun. 2014;50(99):15696–8.
Wang W, Cameron AG, Ke S. Developing fluorescent hyaluronan analogs for hyaluronan studies. Molecules. 2012;17(2):1520–34.
Zhang L, Mummert ME. Development of a fluorescent substrate to measure hyaluronidase activity. Anal Biochem. 2008;379(1):80–5.
Xie H, Zeng F, Wu S. Ratiometric fluorescent biosensor for hyaluronidase with hyaluronan as both nanoparticle scaffold and substrate for enzymatic reaction. Biomacromolecules. 2014;15(9):3383–9.
Huang C, Chiang C, Lin Z, Lee K, Chang H. Bioconjugated gold nanodots and nanoparticles for protein assays based on photoluminescence quenching. Anal Chem. 2008;80(5):1497–504.
Chib R, Raut S, Shah S, Grobelna B, Akopova I, Rich R, et al. Steady state and time resolved fluorescence studies of azadioxatriangulenium (ADOTA) fluorophore in silica and PVA thin films. Dyes Pigments. 2015;117:16–23.
Folmar M, Shtoyko T, Fudala R, Akopova I, Gryczynski Z, Raut S, et al. Metal enhanced fluorescence of Me-ADOTA Cl dye by silver triangular nanoprisms on a gold film. Chem Phys Lett. 2012;531:126–31.
Laursen BW, Sørensen TJ. Synthesis of super stable triangulenium dye. J Org Chem. 2009;74(8):3183–5.
Maliwal BP, Fudala R, Raut S, Kokate R, Sørensen TJ, Laursen BW, et al. Long-lived bright red emitting azaoxa-triangulenium fluorophores. Plos one. 2013;8(5):0063043.
Shtoyko T, Raut S, Rich RM, Sronce RJ, Fudala R, Mason RN, et al. Preparation of plasmonic platforms of silver wires on gold mirrors and their application to surface enhanced fluorescence. ACS Appl Mater Interfaces. 2014;6(21):18780–7.
Sørensen TJ, Laursen BW, Luchowski R, Shtoyko T, Akopova I, Gryczynski Z, et al. Enhanced fluorescence emission of Me-ADOTA by self-assembled silver nanoparticles on a gold film. Chem Phys Lett. 2009;476(1):46–50.
Sørensen TJ, Thyrhaug E, Szabelski M, Luchowski R, Gryczynski I, Gryczynski Z, et al. Azadioxatriangulenium: a long fluorescence lifetime fluorophore for large biomolecule binding assay. Methods Appl Fluoresc. 2013;1(2):025001.
Raut SL, Rich R, Shtoyko T, Bora I, Laursen BW, Sørensen TJ, et al. Sandwich type plasmonic platform for MEF using silver fractals. Nanoscale. 2015;7(42):17729–34.
Bogh SA, Bora I, Rosenberg M, Thyrhaug E, Laursen BW, Sørensen TJ. Azadioxatriangulenium: exploring the effect of a 20 ns fluorescence lifetime in fluorescence anisotropy measurements. Methods Appl Fluoresc. 2015;3(4):045001.
Laursen BW, Krebs FC. Synthesis, structure, and properties of azatriangulenium salts. Chem-A Eur J. 2001;7(8):1773–83.
Bora I, Bogh SA, Santella M, Rosenberg M, Sørensen TJ, Laursen BW. Azadioxatriangulenium: synthesis and photophysical properties of reactive dyes for bioconjugation. Eur J Organ Chem. 2015:6351–6358.
Deng Y, Feng X, Zhou M, Qian Y, Yu H, Qiu X. Investigation of aggregation and assembly of alkali lignin using iodine as a probe. Biomacromolecules. 2011;12(4):1116–25.
Guo Z, Park S, Yoon J, Shin I. Recent progress in the development of near-infrared fluorescent probes for bioimaging applications. Chem Soc Rev. 2014;43(1):16–29.
Ajayaghosh A, Carol P, Sreejith S. A ratiometric fluorescence probe for selective visual sensing of Zn2. J Am Chem Soc. 2005;127(43):14962–3.
Fan J, Hu M, Zhan P, Peng X. Energy transfer cassettes based on organic fluorophores: construction and applications in ratiometric sensing. Chem Soc Rev. 2013;42(1):29–43.
Niu L, Guan Y, Chen Y, Wu L, Tung C, Yang Q. BODIPY-based ratiometric fluorescent sensor for highly selective detection of glutathione over cysteine and homocysteine. J Am Chem Soc. 2012;134(46):18928–31.
Lokeshwar VB, Lokeshwar BL, Pham HT, Block NL. Association of elevated levels of hyaluronidase, a matrix-degrading enzyme, with prostate cancer progression. Cancer Res. 1996;56(3):651–7.
Kuppusamy U, Das N. Inhibitory effects of flavonoids on several venom hyaluronidases. Experientia. 1991;47(11–12):1196–200.
Tung J, Mark GE, Hollis GF. A microplate assay for hyaluronidase and hyaluronidase inhibitors. Anal Biochem. 1994;223(1):149–52.
Acknowledgments
This work was supported by UNTHSC intramural grant RI6120 (R.F.), Sigma Xi grants in aid of research G20141015656984 (R.C.), UNTHSC pre-doctoral bridge grant RI6171 (R.C.), NIH grant R01EB12003 (Z.G.), and NSF grant CBET-1264608 (I.G.). We would like to thank Dr. Andras Lacko and Dr. Nirupama Sabnis for providing us the DU-145 cell line.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 391 kb)
Rights and permissions
About this article
Cite this article
Chib, R., Mummert, M., Bora, I. et al. Fluorescent biosensor for the detection of hyaluronidase: intensity-based ratiometric sensing and fluorescence lifetime-based sensing using a long lifetime azadioxatriangulenium (ADOTA) fluorophore. Anal Bioanal Chem 408, 3811–3821 (2016). https://doi.org/10.1007/s00216-016-9472-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-016-9472-5