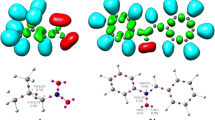
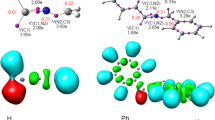
Abstract
Cyclotrisilenes can pursue four types of reaction pathways with unsaturated substrates: π-addition, σ-insertion, exocyclic σ-insertion, and ring-opening reactions. A computational investigation of all these reaction pathways of 1,2,3,3-tetramethyl cyclotrisilene c-Si3Me4 (I) and 1,2-bis(trimethylsilyl)-3,3-dimethyl cyclotrisilene c-Si3Me2(SiMe3)2 (II) with phenylacetylene (R1) and benzaldehyde (R2) is carried out. The reaction pathways are found to be significantly influenced by the substituents attached to the cyclotrisilene ring. Both the π-addition and the σ-insertion reactions proceed with moderate activation energy and high exoergicity, and the electronic nature of the functional group is crucial in deciding the favorable pathway. The exocyclic σ-insertion reactions are found to possess a huge energy barrier, irrespective of the steric and electronic nature of cyclotrisilenes and the substrates. While the course of the reaction and the viability of the ring-opening reaction with phenylacetylene are impacted by the nature of cyclotrisilene, the ring-opening reactions of I and II with benzaldehyde are both highly endoergic.


















Similar content being viewed by others
Data availability
No datasets were generated or analyzed during the current study.
References
Brook AG, Abdesaken F, Gutekunst B et al (1981) A solid silaethene: isolation and characterization. J Chem Soc Chem Commun 4:191–192. https://doi.org/10.1039/c39810000191
West R, Fink MJ, Michl J (1981) Tetramesityldisilene, a stable compound containing a silicon-silicon double bond. Science 214:1343–1344. https://doi.org/10.1126/science.214.4527.1343
Raabe G, Michl J (1985) Multiple bonding to silicon. Chem Rev 85:419–509. https://doi.org/10.1021/cr00069a005
Al-Rubaiey N, Walsh R (1994) Gas-phase kinetic study of the prototype silylene addition reaction SiH2+C2H4 over the temperature range 298–595 K. An example of a third-body mediated association. J Phys Chem 98:5303–5309. https://doi.org/10.1021/j100071a021
Becerra R, Walsh R (1994) Gas-phase kinetic study of the silylene addition reaction to acetylene and acetylene-d2 over the temperature range 291–613 K. Int J Chem Kinet 26:45–60. https://doi.org/10.1002/kin.550260107
Erwin JW, Ring MA, O’Neal HE (1985) Mechanism and kinetics of the silane decomposition in the presence of acetylene and in the presence of olefins. Int J Chem Kinet 17:1067–1083. https://doi.org/10.1002/kin.550171004
Rogers DS, Walker KL, Ring MA, O’Neal HE (1987) Silylene reactions with ethylene and butadiene: mechanism and kinetics. Organometallics 6:2313–2318. https://doi.org/10.1021/om00154a008
Nagase S (1993) Theoretical study of heteroatom-containing compounds. From aromatic and polycyclic molecules to hollow cage clusters. Pure Appl Chem 65:675–682. https://doi.org/10.1351/pac199365040675
Rappoport Z, Apeloig Y (1998) The chemistry of organic silicon compounds. Wiley, New York
Kosa M, Karni M, Apeloig Y (2006) Trisilaallene and the relative stability of Si3H4 isomers. J Chem Theory Comput 2:956–964. https://doi.org/10.1021/ct050154a
Iwamoto T, Kabuto C, Kira M (1999) The first stable cyclotrisilene. J Am Chem Soc 121:886–887. https://doi.org/10.1021/ja983623+
Ichinohe M, Matsuno T, Sekiguchi A (1999) Synthesis, characterization, and crystal structure of cyclotrisilene: a three-membered ring compound with a Si−Si double bond. Angew Chem Int Ed 38:2194–2196. https://doi.org/10.1002/(SICI)1521-3773(19990802)38:15%3c2194::AID-ANIE2194%3e3.0.CO;2-L
Iwamoto T, Tamura M, Kabuto C, Kira M (2000) A stable bicyclic compound with two Si=Si double bonds. Science 290:504–506. https://doi.org/10.1126/science.290.5491.504
Lee VY, Ichinohe M, Sekiguchi A et al (2000) the first three-membered unsaturated rings consisting of different heavier group 14 elements: 1-disilagermirene with a SiSi double bond and its isomerization to a 2-disilagermirene with a SiGe double bond. J Am Chem Soc 122:9034–9035. https://doi.org/10.1021/ja001551s
Göller A, Heydt H, Clark T (1996) σ*-aromaticity of substituted 1H-phosphirenium cations and substituted silacyclopropenes†. J Org Chem 61:5840–5846. https://doi.org/10.1021/jo960387h
Uchiyama K, Nagendran S, Ishida S et al (2007) Thermal and photochemical cleavage of SiSi double bond in tetrasila-1,3-diene. J Am Chem Soc 129:10638–10639. https://doi.org/10.1021/ja0741473
Lee VY, Yasuda H, Sekiguchi A (2007) Interplay of EnE‘3-nC valence isomers (E, E‘ = Si, Ge): bicyclo[1.1.0]butanes with very short bridging bonds and their isomerization to alkyl-substituted cyclopropenes. J Am Chem Soc 129:2436–2437. https://doi.org/10.1021/ja068229n
Leszczyńska K, Abersfelder K, Mix A et al (2012) Reversible base coordination to a disilene. Angew Chem Int Ed 51:6785–6788. https://doi.org/10.1002/anie.201202277
Tsurusaki A, Kamiyama J, Kyushin S (2014) Tetrasilane-bridged bicyclo[4.1.0]heptasil-1(6)-ene. J Am Chem Soc 136:12896–12898. https://doi.org/10.1021/ja507279z
Ichinohe M, Igarashi M, Sanuki K, Sekiguchi A (2005) Cyclotrisilenylium ion: the persilaaromatic compound. J Am Chem Soc 127:9978–9979. https://doi.org/10.1021/ja053202+
Lee VY, Matsuno T, Ichinohe M, Sekiguchi A (2001) Interconversion of cyclotrimetallenes and dihalocyclotrimetallanes consisting of group 14 elements. Heteroat Chem 12:223–226. https://doi.org/10.1002/hc.1036
Tanaka H, Inoue S, Ichinohe M et al (2011) Synthesis and striking reactivity of an isolable tetrasilyl-substituted trisilaallene. Organometallics 30:3475–3478. https://doi.org/10.1021/om200405e
Tsutsui S, Sakamoto K, Kabuto C, Kira M (1998) X-ray crystallographic analysis of a 3-silacyclopropene with electronegative substituents on silicon. Organometallics 17:3819–3821. https://doi.org/10.1021/om980207p
Präsang C, Scheschkewitz D (2016) Reactivity in the periphery of functionalised multiple bonds of heavier group 14 elements. Chem Soc Rev 45:900–921. https://doi.org/10.1039/C5CS00720H
Fischer RC, Power PP (2010) π-Bonding and the lone pair effect in multiple bonds involving heavier main group elements: developments in the new millennium. Chem Rev 110:3877–3923. https://doi.org/10.1021/cr100133q
Ya. Lee V, Sekiguchi A (2010) Organometallic compounds of low-coordinate Si, Ge, Sn and Pb: from phantom species to stable compounds. J. Wiley & Sons, Ltd,
Pintér B, Olasz A, Petrov K, Veszprémi T (2007) Cyclotrimetallenes: bridged and distorted structures. Organometallics 26:3677–3683. https://doi.org/10.1021/om700267j
Cowley MJ, Ohmori Y, Huch V et al (2013) Carbonylation of cyclotrisilenes. Angew Chem Int Ed 52:13247–13250. https://doi.org/10.1002/anie.201307450
Ohmori Y, Ichinohe M, Sekiguchi A et al (2013) Functionalized cyclic disilenes via ring expansion of cyclotrisilenes with isocyanides. Organometallics 32:1591–1594. https://doi.org/10.1021/om400054u
Lee VY, Gapurenko OA, Miyazaki S et al (2015) From a Si3-cyclopropene to a Si3S-bicyclo[1.1.0]butane to a Si3S-cyclopropene to a Si3S2-Bicyclo[1.1.0]butane: back-and-forth, and in-between. Angew Chem 127:14324–14328. https://doi.org/10.1002/ange.201506625
Cowley MJ, Huch V, Rzepa HS, Scheschkewitz D (2013) Equilibrium between a cyclotrisilene and an isolable base adduct of a disilenyl silylene. Nat Chem 5:876–879. https://doi.org/10.1038/nchem.1751
Zhao H, Leszczyńska K, Klemmer L et al (2018) Disilenyl silylene reactivity of a cyclotrisilene. Angew Chem Int Ed 57:2445–2449. https://doi.org/10.1002/anie.201711833
Lee VY, Ichinohe M, Sekiguchi A (2001) Reaction of 1-disilagermirene with benzaldehyde: an unexpected combination of cycloaddition and insertion pathways. Chem Lett 30:728–729. https://doi.org/10.1246/cl.2001.728
Lee VY, Miyazaki S, Yasuda H, Sekiguchi A (2008) Isomeric metamorphosis: Si3E (E = S, Se, and Te) bicyclo[1.1.0]butane and cyclobutene. J Am Chem Soc 130:2758–2759. https://doi.org/10.1021/ja800111r
Ichinohe M, Matsuno T, Sekiguchi A (2001) Reaction of cyclotrisilene with phenylacetylene: an unusual product with a bicyclo[3.2.0]hepta-3,6-diene skeleton. Chem Commun. https://doi.org/10.1039/b008375p
Yildiz CB (2020) A DFT study on the oxidation of cyclotrisilene by nitrous oxide: the σ- and π-bonds reactivity. Theor Chem Acc 139:18. https://doi.org/10.1007/s00214-019-2540-0
Frisch MJ, Trucks GW, Schlegel HB, et al (2016) G16_C01. Gaussian 16, Revision C.01, Gaussian, Inc., Wallin
Zhao Y, Truhlar DG (2008) The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor Chem Acc 120:215–241. https://doi.org/10.1007/s00214-007-0310-x
McLean AD, Chandler GS (1980) Contracted Gaussian basis sets for molecular calculations. I. Second row atoms, Z =11–18. J Chem Phys 72:5639–5648. https://doi.org/10.1063/1.438980
Krishnan R, Binkley JS, Seeger R, Pople JA (1980) Self-consistent molecular orbital methods. XX. A basis set for correlated wave functions. J Chem Phys 72:650–654. https://doi.org/10.1063/1.438955
Peng C, Bernhard Schlegel H (1993) Combining synchronous transit and quasi-newton methods to find transition states. Isr J Chem 33:449–454. https://doi.org/10.1002/ijch.199300051
Marenich AV, Cramer CJ, Truhlar DG (2009) Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J Phys Chem B 113:6378–6396. https://doi.org/10.1021/jp810292n
Acknowledgements
The authors are grateful to Rashtriya Uchchatar Shiksha Abhiyan (RUSA) and University Grants Commission (UGC) for the financial support. AK thanks Kerala State Council for Science, Technology and Environment (KSCSTE), for a fellowship.
Author information
Authors and Affiliations
Contributions
Major part of the work has been done by A. K. and J. J. M. J. M. wrote the main manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kizhuvedath, A., Mallikasseri, J.J. & Mathew, J. Unraveling the reaction pathways of cyclotrisilenes: a computational analysis. Theor Chem Acc 143, 23 (2024). https://doi.org/10.1007/s00214-024-03099-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-024-03099-9