Abstract
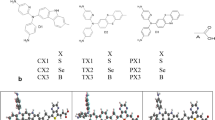
Meeting the requirements and developments of modern society will be unachievable without a sustainable source of energy. However, dye sensitized solar cell (DSSC) has been found worthy to produce reliable energy source. Recently, researchers have inputted great effort toward the modulation of organic dyes to achieve highly efficient dye sensitizers for solar cell purposes. Herein, D-π-A architectural design was employed to generate ten phenoxazine-based dye engineered with five different thiophene π-linkers and two acceptor units. Density functional theory (DFT) and time-dependent DFT were used to optimize the molecules in order to cognize their intramolecular charge transport, optoelectronic properties, light-harvesting efficiency (LHE) and open-circuit voltage (Voc). DFT conceptual was engaged to elucidate the chemical reactivity parameters of the photosensitizers. Among the π-linkers employed, 2,6-diethenylbisthieno[3,2-b:2′,3′-d]thiophene (D3) remarkably improved the intramolecular charge transfer, thus generated an exceptional HOMO/LUMO energy gap (Eg), strong bathochromic shift, suitable LHE and notable Voc. The qualities of the dyes were further enhanced by replacing 2-cyano-2-pyran-4-ylidene-acetic acid with cyanoacrylic acid acceptor moiety. Comparison of these systems with other reported molecules reveals that majority of the simulated poses improved properties that are recommendable for solar cells applications.





Similar content being viewed by others
References
Balat M (2005) Usage of energy sources and environmental problems. Energy Explor Exploit. https://doi.org/10.1260/0144598054530011
Haradhan KM (2018) Acid rain is a local environment pollution but global concern. Open Sci J Anal Chem 3:47–55
Frederica P (2018) Pollution from fossil-fuel combustion is the leading environmental threat to global pediatric health and equity: solutions exist. Int J Environ Res Public Health. https://doi.org/10.3390/ijerph15010016
Birel O (2015) An overview on the some phenothiazine derivative molecules used in organic dye-sensitized solar cells. EJOVOC. https://doi.org/10.17339/EJOVOC.61556
Tao CS, Jiang J, Tao M (2011) Natural resource limitations to terawatt-scale solar cells. Solar Energy Mater Sol Cells. https://doi.org/10.1016/j.solmat.2011.06.013
Zhang S, Yang X, Numata Y, Han L (2013) Highly efficient dye-sensitized solar cells: progress and future challenges. Energy Environ Sci. https://doi.org/10.1039/C3EE24453A
Sharma K, Sharma V, Sharma SS (2018) Dye-sensitized solar cells: fundamentals and current status. Nanoscale Res Lett. https://doi.org/10.1186/s11671-018-2760-6
Grätzel M (2009) Recent advances in sensitized mesoscopic solar cell. Acc Chem Res. https://doi.org/10.1021/ar900141y
Yen YS, Chou HH, Chen YC, Hsu CY, Lin JT (2012) Recent developments in molecule-based organic materials for dye-sensitized solar cells. J Mater Chem. https://doi.org/10.1039/C2JM30362K
Seo KD, Choi IT, Park YG, Kang S, Lee JY, Kim HK (2012) Novel D-A-π-A coumarin dyes containing low band-gap chromophores for dye-sensitized solar cells. Dyes Pigm. https://doi.org/10.1016/j.dyepig.2012.02.015
Wu Y, Marszalek M, Zakeeruddin SM, Zhang Q, Tian H, Gratzel M, Zhu W (2012) High-conversion-efficiency organic dye-sensitized solar cells: molecular engineering on D-A-π-A featured organic indoline dyes. Energy Environ Sci. https://doi.org/10.1039/C2EE22108J
Zhang X, Gou F, Shi J, Gao H, Zhu Z, Jing H (2016) Molecular engineering of new phenothiazine-based D-A-π-A dyes for dye-sensitized solar cells. RSC Adv. https://doi.org/10.1039/C6RA20769C
Li C, Yum JH, Moon SJ, Herrmann A, Eickemeyer F, Pschirer NG, Erk P, Schoneboom J, Mullen K, Gratzel M, Nazeeruddin MK (2008) An improved perylene sensitizer for solar cell applications. Chemsuschem. https://doi.org/10.1002/cssc.200800068
Wan Z, Jia C, Duan Y, Chen X, Li Z, Lin Y (2014) Novel organic sensitizers containing dithiafulvenyl units as additional donors for efficient dye-sensitized solar cells. RSC Adv. https://doi.org/10.1039/C4RA04782F
Peng B, Yang S, Li L, Cheng F, Chen JA (2010) Density functional theory and time-dependent density functional theory investigation on the anchor comparison of triarylamine-based dyes. J Chem Phys doi. https://doi.org/10.1063/1.3292639
Tian H, Yang X, Pan J, Chen R, Liu M, Zhang Q, Hagfeldt A, Sun L (2008) A triphenylamine dye model for the study of intramolecular energy transfer and charge transfer in dye sensitized solar cells. Adv Funct Mater. https://doi.org/10.1002/adfm.200800516
Mehmood U, Hussein IA, Harrabi K, Ahmed S (2015) Density functional theory study on the electronic structures of oxadiazole based dyes as photosensitizer for dye sensitized solar cells. Adv Mater Sci Eng. https://doi.org/10.1155/2015/286730
Kim D, Lee JK, Kang SO, Ko J (2007) Molecular engineering of organic dyes containing N-aryl carbazole moiety for solar cell. Tetrahedron. https://doi.org/10.1016/j.tet.2006.12.082
Tan H, Pan C, Wang G, Wu Y, Zhang Y, Yu G, Zhang MA (2014) Comparative study on properties of two phenoxazine-based dyes for dye-sensitized solar cells. Dyes Pigm. https://doi.org/10.1016/j.dyepig.2013.09.039
Chermahini ZJ, Chermahini AN (2017) Theoretical study on the bridge comparison of TiO2 nanoparticle sensitizers based on phenoxazine in dye-sensitized solar cells. Theor Chem Acc. https://doi.org/10.1007/s00214-017-2063-5
Numata Y, Islam A, Chen H, Han L (2012) Aggregation-free branch-type organic dye with a twisted molecular architecture for dye-sensitized solar cells. Energy Environ Sci. https://doi.org/10.1039/C2EE22506A
Karlsson KM, Jiang X, Eriksson SK, Gabrielsson E, Rensmo H, Hagfeldt A, Sun L (2011) Phenoxazine dyes for dye-sensitized solar cells: relationship between molecular structure and electron lifetime. Chem Eur J. https://doi.org/10.1002/chem.201003730
Lee W, Yuk SB, Choi J, Kim HJ, Kim HW, Kim SH, Kim B, Ko MJ, Kim JP (2014) The effects of the number of anchoring groups and N-substitution on the performance of phenoxazine dyes in dye-sensitized solar cells. Dyes Pigm. https://doi.org/10.1016/j.dyepig.2013.10.005
Semire B, Oyebamiji A, Odunola OA (2016) Design of (2Z)-2-cyano-2-[2-[(E)-2-[5-[(E)-2-(4-dimethylaminophenyl)vinyl]-2-thienyl]vinyl]pyran-4-ylidene]acetic acid derivatives as D- π-A dye sensitizers in molecular photovoltaics: a density functional theory approach. Res Chem Intermed. https://doi.org/10.1007/s11164-015-2303-z
Semire B, Oyebamiji A, Odunola OA (2017) Tailoring of energy levels in (2Z)-2-cyano-2-[2-[(E)-2-[2-[(E)-2-(ptolyl)vinyl]thieno[3,2-b]thiophen-5-yl]vinyl]pyran-4-ylidene]acetic acid derivatives via conjugate bridge and fluorination of acceptor units for effective D–π–A dye-sensitized solar cells: DFT–TDDFT approach. Res Chem Intermed. https://doi.org/10.1007/s11164-016-2735-0
Lee C, Yang W, Parr RG (1988) Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B. https://doi.org/10.1103/PhysRevB.37.785
Li W, Wang J, Chen J, Bai FQ, Zhang HX (2014) Theoretical investigation of triphenylamine-based sensitizers with different pi-linkers for DSSC. Spectrochim Acta A. https://doi.org/10.1016/j.saa.2013.09.080
Delgado-Montiel T, Baldenebro-López J, Soto-Rojo R, Glossman-Mitnik D (2016) Quantum chemical study of the effect of π-bridge on the optical and electronic properties of sensitizers for DSSCs incorporating dioxythiophene and thiophene units. Theor Chem Acc. https://doi.org/10.1007/s00214-016-1989-3
Bourass M, Benjelloun AT, Hamidi M, Benzakour M, Mcharfi M, Sfaira M, Serein SF, Lere-Porte JP, Sotiropoulos JM, Bouzzine SM, Bouachrine M, Saudi J (2013) DFT theoretical investigations of p-conjugated molecules based on thienopyrazine and different acceptor moieties for organic photovoltaic cells. J Saudi Chem Soc. https://doi.org/10.1016/j.jscs.2013.01.003
Andrzej E (2010) TDDFT study of absorption spectrum of ketocyanine dye complexes with metal ions: explicit solvent model. Theor Chem Acc. https://doi.org/10.1007/s00214-010-0791-x
Mohajeri A, Omidvar A, Setoodeh H (2018) Fine structural tuning of thieno [3, 2-b] pyrrole donor for designing banana-shaped semiconductors relevant to organic field effect transistors. J Chem Inf Model. https://doi.org/10.1021/acs.jcim.8b00738
Semire B, Oyebamiji A, Odunola AO (2020) Electronic properties’ modulation of D-A–A via fluorination of 2-cyano-2-pyran-4-ylidene-acetic acid acceptor unit for efficient DSSCs: DFT-TDDFT approach. Sci Afr. https://doi.org/10.1016/j.sciaf.2020.e00287
Jacquemin D, Perpete EA, Ciofini I, Adamo C (2009) Accurate simulation of optical properties in dyes. Acc Chem Res. https://doi.org/10.1021/ar800163d
Lu T, Chen F (2012) Multiwfn: a multifunctional wavefunction analyzer. J Comput Chem. https://doi.org/10.1002/jcc.22885
Delgado-Montiel T, Baldenebro-López J, Soto-Rojo R, Glossman-Mitnik D (2020) Theoretical study of the effect of π-bridge on optical and electronic properties of carbazole-based sensitizers for DSSCs. Molecules. https://doi.org/10.3390/molecules25163670
Spartan 14, Wavefunction, Inc. Irvine CA 92612, USA.
Vuai SA, Khalfan MS, Babu NS (2021) DFT and TD-DFT studies for optoelectronic properties of coumarin based donor-π-acceptor (D-π-A) dyes: applications in dye-sensitized solar cells (DSSCS). Heliyon. https://doi.org/10.1016/j.heliyon.2021.e08339
Hachi M, Slimi A, Fitri A, Benjelloun AT, Benzakour M, Mcharfi M, Khenfouch M, Zorkani I, Bouachrine M (2021) Theoretical design and characterization of D-A1-A based organic dyes for efficient DSSC by altering promising acceptor (A1) moiety. J Photochem Photobiol. https://doi.org/10.1016/j.jphotochem.2020.113048
Deogratias G, Seriani N, Pogrebnaya T, Pogrebnoi A (2020) Tuning optoelectronic properties of triphenylamine based dyes through variation of pi-conjugated units and anchoring groups: A DFT/TD-DFT investigation. J Mol Graph. https://doi.org/10.1016/j.jmgm.2019.107480
Souilah M, Hachi M, Fitri A, Benjelloun AT, El Khattabi S, Benzakour M, Mcharfi M, Zgou H (2021) Coumarin-based D–π–A dyes for efficient DSSCs: DFT and TD-DFT study of the π-spacers influence on photovoltaic properties. Res Chem Intermed. https://doi.org/10.1007/s11164-020-04302-9
Li M, Kou L, Diao L, Zhang Q, Li Z, Wu Q, Lu W, Pan D (2015) Theoretical study of acene-bridged dyes for dye-sensitized solar cells. J Phys Chem. https://doi.org/10.1021/acs.jpca.5b00798
Fu JJ, Duan YA, Zhang JZ, Guo MS, Liao Y (2014) Theoretical investigation of novel phenothiazine-based D-π-A conjugated organic dyes as dye-sensitizer in dye-sensitized solar cells. Comput Theor Chem. https://doi.org/10.1016/j.comptc.2014.07.008
Sang-aroon W, Saekow S, Amornkitbamrung V (2012) Density functional theory study on the electronic structure of Monascus dyes as photosensitizer for dye-sensitized solar cells. J Photochem Photobiol. https://doi.org/10.1016/j.jphotochem.2012.03.014
Bouzayen N, Mbarek M, Alimi K (2015) Solvent effects on optical and electronic properties of carbazole benzothiazole based bipolar compound: TD-DFT/PCM approach. Mor J Chem https://doi.org/10.48317/IMIST.PRSM/morjchem-v3i1.2259
Afolabi SO, Semire B, Idowu MA (2021) Electronic and optical properties’ tuning of phenoxazine-based D-A2-π-A1 organic dyes for dye-sensitized solar cells. DFT/TDDFT investigations. Heliyo. https://doi.org/10.1016/j.heliyon.2021.e06827
Kumar MD, Rajesh P, Dharsini RP, Inban ME (2020) Molecular geometry, NLO, MEP, HOMO-LUMO and Mulliken charges of substituted piperidine phenyl hydrazines by using density functional theory. Asian J Chem https://doi.org/10.14233/ajchem.2020.22444
Raftani M, Abram T, Bennani MN, Bouachrine M (2020) Theoretical study of new conjugated compounds with a low bandgap for bulk heterojunction solar cells: DFT and TD-DFT study. Results Chem. https://doi.org/10.1016/j.rechem.2020.100040
Miar M, Shiroudi A, Pourshamsian K, Oliaey AR, Hatamjafari F (2021) Theoretical investigations on the HOMO–LUMO gap and global reactivity descriptor studies, natural bond orbital, and nucleus-independent chemical shifts analyses of 3-phenylbenzo [d] thiazole-2 (3 H)-imine and its para-substituted derivatives: Solvent and substituent effects. J Chem Res. https://doi.org/10.1177/1747519820932091
Fitri A, Benjelloun AT, Benzakour M, McHarfi M, Hamidi M, Bouachrine M (2014) Theoretical investigation of new thiazolothiazole-based D-π-A organic dyes for efficient dye-sensitized solar cell. Spectrochim Acta Part A. https://doi.org/10.1016/j.saa.2014.01.052
Kurashige Y, Nakajima T, Kurashige V, Hirao K, Nishikitani YJ (2007) Theoretical investigation of the excited states of coumarin dyes for dye-sensitized solar cells. J Phys Chem A. https://doi.org/10.1021/jp0720688
Afolabi SO, Semire B, Akiode OK, Afolabi TA, Adebayo GA, Idowu MA (2020) Design and theoretical study of phenothiazine-based low bandgap dye derivatives as sensitizers in molecular photovoltaics. Opt Quantum Electron. https://doi.org/10.1007/s11082-020-02600-5
Tian H, Yang X, Pan J, Chen R, Liu M, Zhang Q, Hagfeldt A, Sun LA (2008) Triphenylamine dye model for the study of intramolecular energy transfer and charge transfer in dye sensitized solar cells. Adv Funct Mater. https://doi.org/10.1002/adfm.200800516
Maurano A, Hamilton R, Shuttle CG, Ballantyne AM, Nelson J, O’Regan B, Zhang W, McCulloch I, Azimi H, Morana M, Brabec CJ, Durrant JR (2010) Recombination dynamics as a key determinant of open circuit voltage in organic bulk heterojunction solar cells: a comparison of four different donor polymers. Adv Mater. https://doi.org/10.1002/adma.201002360
Honsberg CB, Bowden SG (2019) Photovoltaics education website. PV Education. https://www.pveducation.org/pvcdrom/welcome-to-pvcdrom/instructions. Accessed 4 January 2022
Wazzan NA (2019) A DFT/TDDFT investigation on the efficiency of novel dyes with ortho-fluorophenyl units (A1) and incorporating benzotriazole/benzothiadiazole/ phthalimide units (A2) as organic photosensitizers with D-A2–π–A1 configuration for solar cell applications. J Comput Electron. https://doi.org/10.1007/s10825-019-01308-4
Tomas DM, Rody SR, Jesus BL, Daniel GM (2019) Theoretical study of the effect of different π bridges including an azomethine group in triphenylamine-based dye for dye-sensitized solar cells. Molecules. https://doi.org/10.3390/molecules24213897
Parr RG, Szentpaly L, Liu S, Am J (1999) Electrophilicity Index. J Am Chem Soc. https://doi.org/10.1021/ja983494x
Domingo LR, Ríos-Gutierrez M, Pere P (2016) Applications of the conceptual density functional theory indices to organic chemistry reactivity. Molecules. https://doi.org/10.3390/molecules21060748
Gazquez JL, Cedillo A, Vela A (2007) Electrodonating and electroaccepting powers. J Phys Chem A. https://doi.org/10.1021/jp065459f
Sun C, Li Y, Song P, Ma F (2016) An experimental and theoretical investigation of the electronic structures and photoelectrical properties of ethyl red and carminic acid for DSSC application. Materials. https://doi.org/10.3390/ma9100813
Acknowledgements
The contributions of staff from the Department of Chemistry, Federal University of Agriculture, Abeokuta, Ogun State, Nigeria, are well appreciated. Authors thank the Department of Pure and Applied Chemistry, LAUTECH, Ogbomoso, for the provision of facilities to carry out the research.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of interest
Authors declare that there are no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Afolabi, S.O., Semire, B., Akiode, O.K. et al. Quantum study on the optoelectronic properties and chemical reactivity of phenoxazine-based organic photosensitizer for solar cell purposes. Theor Chem Acc 141, 22 (2022). https://doi.org/10.1007/s00214-022-02882-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-022-02882-w