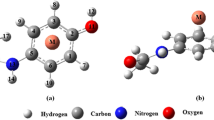
Abstract
The stability constants of the non-steroidal anti-inflammatory drug indomethacin mononuclear copper complexes, Cu(Indo)+ and Cu(Indo)2, were calculated by thermodynamic cycles in the frame of density functional theory. The BH&HLYP functional with LANL2DZ and Def2SVP basis set for copper ion and lighter atoms, respectively, were used to perform the search of minimums, and to consider thermal corrections in gas phase. For the minimums found, electronic energies were evaluated by performing single point calculations, using the basis set LANL2TZ+f for copper ion and the 6-311++G(2d,2p) for the rest. To include the continuum contribution to the solvation energy, the solvation model based on density was applied. Cluster/continuum model yields acceptable results in predicting solvation energies of Cu(II) ion and proton with deviations of 4.2 and 4.7 kcal/mol at the worked level of theory. Cluster/continuum calculations of Cu(Indo)+ and Cu(Indo)2 complexes stability constants, reported as logβ, predict 1.65 and − 3.17 in water and 15.9 and 26.7 in ethanol. We also apply the ion-exchange thermodynamic cycle, which aims error cancelation through structural similarity between species in both sides of chemical reactions. Encouraging results are obtained for Cu(Indo)2 stability constant in ethanol, logβ = 16.37, differing just 2.32 units from the experimental value. Structural features and charge distribution, of the species involved in the complexation reaction, are discussed to rationalize the performing of thermodynamic cycles in predicting complexation stability constants.















Similar content being viewed by others
References
Tagliati CA, Kimura E, Nothenberg MS et al (1999) Pharmacokinetic profile and adverse gastric effect of zinc-piroxicam in rats. Gen Pharmacol 33:67–71. https://doi.org/10.1016/S0306-3623(98)00267-5
Weder JE, Dillon CT, Hambley TW et al (2002) Copper complexes of non-steroidal anti-inflammatory drugs: an opportunity yet to be realized. Coord Chem Rev 232:95–126. https://doi.org/10.1016/S0010-8545(02)00086-3
Pantovic A, Bosnjak M, Arsikin K et al (2016) In vitro antiglioma action of indomethacin is mediated via AMP-activated protein kinase/mTOR complex 1 signalling pathway. Int J Biochem Cell Biol 83:84–96. https://doi.org/10.1016/j.biocel.2016.12.007
Brunelli C, Amici C, Angelini M et al (2012) The non-steroidal anti-inflammatory drug indomethacin activates the eIF2α kinase PKR, causing a translational block in human colorectal cancer cells. Biochem J 443:379–386. https://doi.org/10.1042/BJ20111236
Eli Y, Przedecki F, Levin G et al (2001) Comparative effects of indomethacin on cell proliferation and cell cycle progression in tumor cells grown in vitro and in vivo. Biochem Pharmacol 61:565–571
Hojka-Osinska A, Ziolo E, Rapak A (2012) Combined treatment with fenretinide and indomethacin induces AIF-mediated, non-classical cell death in human acute T-cell leukemia Jurkat cells. Biochem Biophys Res Commun 419:590–595. https://doi.org/10.1016/j.bbrc.2012.02.092
Morecki S, Yacovlev E, Gelfand Y et al (2000) Induction of antitumor immunity by indomethacin. Cancer Immunol Immunother 48:613–620
Jukić MK, Luetić AT, Skudar-Lukinović V et al (2010) The antimetastatic effect of macrophages restored by indomethacin: concomitant tumor immunity model. Coll Antropol 34:899–904
Levin G, Kariv N, Khomiak E, Raz A (2000) Indomethacin inhibits the accumulation of tumor cells in mouse lungs and subsequent growth of lung metastases. Chemotherapy 46:429–437
Bigda J, Mysliwski A (1998) Indomethacin inhibits kidney metastasis in Bomirski melanoma-bearing hamsters, and modulates natural killer cytotoxic activity of tumor hosts in vivo and in vitro. Anticancer Res 18:3549–3554
Crisponi G, Nurchi VM, Fanni D et al (2010) Copper-related diseases: from chemistry to molecular pathology. Coord Chem Rev 254:876–889. https://doi.org/10.1016/j.ccr.2009.12.018
Bonin AM, Yáñez JA, Fukuda C et al (2010) Inhibition of experimental colorectal cancer and reduction in renal and gastrointestinal toxicities by copper-indomethacin in rats. Cancer Chemother Pharmacol 66:755–764. https://doi.org/10.1007/s00280-009-1220-5
Dillon CT, Hambley TW, Kennedy BJ et al (2003) Gastrointestinal toxicity, antiinflammatory activity, and superoxide dismutase activity of copper and zinc complexes of the antiinflammatory drug indomethacin. Chem Res Toxicol 16:28–37. https://doi.org/10.1021/tx020078o
Tarushi A, Raptopoulou CP, Psycharis V et al (2014) Structure and biological perspectives of Cu(II)-indomethacin complexes. J Inorg Biochem 140:185–198. https://doi.org/10.1016/j.jinorgbio.2014.07.006
Leggett DJ, McBryde WAE (1975) General computer program for the computation of stability constants from absorbance data. Anal Chem 47:1065–1070. https://doi.org/10.1021/ac60357a046
Rodríguez-Laguna N, Reyes-García LI, Moya-Hernández R et al (2016) Chemical speciation of the system Cu(II)-indomethacin in ethanol and water by UV–Vis spectrophotometry. J Chem. https://doi.org/10.1155/2016/9804162
Gutten O, Beššeová I, Rulíšek L (2011) Interaction of metal ions with biomolecular ligands: How accurate are calculated free energies associated with metal ion complexation? J Phys Chem A 115:11394–11402. https://doi.org/10.1021/jp205442p
Tomasi J, Persico M (1994) Molecular interactions in solution: an overview of methods based on continuous distributions of the solvent. Chem Rev 94:2027–2094. https://doi.org/10.1021/cr00031a013
Chipman DM (2003) Anion electric field is related to hydration energy. J Chem Phys 118:9937–9942. https://doi.org/10.1063/1.1572454
Camaioni DM, Dupuis M, Bentley J (2003) Theoretical characterization of oxoanion, XO n− m , solvation. J Phys Chem A 107:5778–5788. https://doi.org/10.1021/jp0343537
Chipman DM, Chen F (2006) Cation electric field is related to hydration energy. J Chem Phys. https://doi.org/10.1063/1.2180784
Bryantsev VS, Diallo MS, Goddard WA III (2008) Calculation of solvation free energies of charged solutes using mixed cluster/continuum models. J Phys Chem B 112:9709–9719. https://doi.org/10.1021/jp802665d
Almerindo GI, Tondo DW, Pliego JR (2004) Ionization of organic acids in dimethyl sulfoxide solution: a theoretical Ab initio calculation of the pKa using a new parametrization of the polarizable continuum model. J Phys Chem A 108:166–171. https://doi.org/10.1021/jp0361071
Mujika JI, Mercero JM, Lopez X (2003) A theoretical evaluation of the pKa for twisted amides using density functional theory and dielectric continuum methods. J Phys Chem A 107:6099–6107. https://doi.org/10.1021/jp035228y
Lopez X, Schaefer M, Dejaegere A, Karplus M (2002) Theoretical evaluation of pKa in phosphoranes: implications for phosphate ester hydrolysis. J Am Chem Soc 124:5010–5018. https://doi.org/10.1021/ja011373i
Dong H, Du H, Qian X (2008) Theoretical prediction of pKa values for methacrylic acid oligomers using combined quantum mechanical and continuum solvation methods. J Phys Chem A 112:12687–12694
Ding F, Smith JM, Wang H (2009) First-principles calculation of pKa values for organic acids in nonaqueous solution. J Org Chem 74:2679–2691. https://doi.org/10.1021/jo802641r
Gómez-Bombarelli R, González-Pérez M, Pérez-Prior MT et al (2009) Computational study of the acid dissociation of esters and lactones. A case study of diketene. J Org Chem 74:4943–4948. https://doi.org/10.1021/jo900645h
Fu Y, Liu L, Li R-Q et al (2004) First-principle predictions of absolute pKa’s of organic acids in dimethyl sulfoxide solution. J Am Chem Soc 126:814–822. https://doi.org/10.1021/ja0378097
Namazian M, Kalantary-Fotooh F, Noorbala MR et al (2006) Møller-Plesset perturbation theory calculations of the pKa values for a range of carboxylic acids. J Mol Struct Theochem 758:275–278. https://doi.org/10.1016/j.theochem.2005.10.024
Toth AM, Liptak MD, Phillips DL, Shields GC (2001) Accurate relative pKa calculations for carboxylic acids using complete basis set and Gaussian-n models combined with continuum solvation methods. J Chem Phys 114:4595–4606. https://doi.org/10.1063/1.1337862
Caballero NA, Melendez FJ, Muñoz-Caro C, Niño A (2006) Theoretical prediction of relative and absolute pKa values of aminopyridines. Biophys Chem 124:155–160. https://doi.org/10.1016/j.bpc.2006.06.007
Magill AM, Cavell KJ, Yates BF (2004) Basicity of nucleophilic carbenes in aqueous and nonaqueous solvents—theoretical predictions. J Am Chem Soc 126:8717–8724. https://doi.org/10.1021/ja038973x
Takano Y, Houk KN (2005) Benchmarking the conductor-like polarizable continuum model (CPCM) for aqueous solvation free energies of neutral and ionic organic molecules. J Chem Theory Comput 1:70–77. https://doi.org/10.1021/ct049977a
Marenich AV, Cramer CJ, Truhlar DG (2009) Universal solvation model based on the generalized born approximation with asymmetric descreening. J Chem Theory Comput 5:2447–2464. https://doi.org/10.1021/ct900312z
Parr RG, Yang W (1989) Density-functional theory of atoms and molecules. Oxford University Press, New York
Lee C, Yang W, Parr RG (1988) Development of the Colle–Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37:785–789. https://doi.org/10.1103/PhysRevB.37.785
Becke AD (1993) A new mixing of Hartree–Fock and local density-functional theories. J Chem Phys 98:1372–1377. https://doi.org/10.1063/1.464304
Galván-García EA, Agacino-Valdés E, Franco-Pérez M, Gómez-Balderas R (2017) [Cu(H2O)n]2+ (n = 1–6) complexes in solution phase: a DFT hierarchical study. Theor Chem Acc 136:29. https://doi.org/10.1007/s00214-017-2056-4
Møller C, Plesset MS (1934) Note on an approximation treatment for many-electron systems. Phys Rev 46:618–622. https://doi.org/10.1103/PhysRev.46.618
Rios-Font R, Sodupe M, Rodriguez-Santiago L, Taylor PR (2010) The role of exact exchange in the description of Cu2+–(H2O)n (n = 1–6) complexes by means of DFT methods. J Phys Chem A 114:10857–10863. https://doi.org/10.1021/jp105376s
Hay PJ, Wadt WR (1985) Ab initio effective core potentials for molecular calculations. Potentials for the transition metal atoms Sc to Hg. J Chem Phys 82:270–283. https://doi.org/10.1063/1.448799
Hay PJ, Wadt WR (1985) Ab initio effective core potentials for molecular calculations. Potentials for K to Au including the outermost core orbitals. J Chem Phys 82:299–310. https://doi.org/10.1063/1.448975
Wadt WR, Hay PJ (1985) Ab initio effective core potentials for molecular calculations. Potentials for main group elements Na to Bi. J Chem Phys 82:284–298. https://doi.org/10.1063/1.448800
Weigend F, Ahlrichs R (2005) Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: design and assessment of accuracy. Phys Chem Chem Phys 7:3297–3305. https://doi.org/10.1039/b508541a
Roy LE, Hay PE, Martin RL (2008) Revised Basis Sets for the LANL Effective Core Potentials. J Chem Theory Comput 4:1029–1031. https://doi.org/10.1021/ct8000409
Marenich AV, Cramer CJ, Truhlar DG (2009) Universal solvation model based on solute electron density and a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J Phys Chem B 113:6378–6396. https://doi.org/10.1021/jp810292n
Gaussian 09, Revision D.01, Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman, JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA Jr, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Keith T, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross, JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas Ö, Foresman, JB, Ortiz JV, Cioslowski J, Fox DJ. (2009) Gaussian Inc., Wallingford CT
Dennington R, Keith T, Millam J (2009) GaussView, Version 5. Semichem Inc., Shawnee Mission. KS Semichem Inc
Kelly CP, Cramer CJ, Truhlar DG (2005) SM6: a density functional theory continuum Solvation Model for calculating aqueous solvation free energies of neutrals, ions, and solute-water clusters. J Chem Theory Comput 1:1133–1152. https://doi.org/10.1021/ct050164b
Kelly CP, Cramer CJ, Truhlar DG (2006) Aqueous solvation free energies of ions and ion–water clusters based on an accurate value for the absolute aqueous solvation free energy of the proton. J Phys Chem B 110:16066–16081. https://doi.org/10.1021/jp063552y
Tissandier MD, Cowen KA, Feng WY et al (1998) The proton’s absolute aqueous enthalpy and gibbs free energy of solvation from cluster–ion solvation data. J Phys Chem A 102:7787–7794. https://doi.org/10.1021/jp982638r
Camaioni DM, Schwerdtfeger CA (2005) Comment on “accurate experimental values for the free energies of hydration of H+, OH–, and H3O+”. J Phys Chem A 109:10795–10797. https://doi.org/10.1021/jp054088k
Reed AE, Weinstock RB, Weinhold F (1985) Natural population analysis. J Chem Phys 83:735–746. https://doi.org/10.1063/1.449486
Kay BD, Castleman AW (1985) Molecular beam electric deflection study of the hydrogen-bonded water, methanol, and ethanol clusters (H2O)N, (CH3OH)N, and (C2H5OH)N. J Phys Chem 89:4867–4868. https://doi.org/10.1021/j100268a041
Hu YJ, Fu HB, Bernstein ER (2006) Infrared plus vacuum ultraviolet spectroscopy of neutral and ionic ethanol monomers and clusters. J Chem Phys. doi 10(1063/1):2357952
Umer M, Kopp WA, Leonhard K (2015) Efficient yet accurate approximations for ab initio calculations of alcohol cluster thermochemistry. J Chem Phys. doi https://doi.org/10.1063/1.4936406
Descroix S, Varenne A, Adamo C, Gareil P (2004) Capillary electrophoresis of inorganic anions in hydro-organic media: influence of ion-pairing and solvation phenomena. J Chromatogr A 1032:149–158. https://doi.org/10.1016/j.chroma.2003.11.070
Fini A, Fazio G, Feroci G (1995) Solubility and solubilization properties of non-steroidal anti-inflammatory drugs. Int J Pharm 126:95–102. https://doi.org/10.1016/0378-5173(95)04102-8
Rappé AKK, Casewit CJJ, Colwell KSS et al (1992) UFF, a full periodic table force field for molecular mechanics and molecular dynamics simulations. J Am Chem Soc 114:10024–10035. https://doi.org/10.1021/ja00051a040
Acknowledgements
Raúl Flores acknowledges DGAPA-UNAM for the postdoctoral fellowship. LIR-G acknowledges to CONACyT for the PhD scholarship. The authors gratefully acknowledge the computing time granted by LANCAD and CONACYT on the supercomputer Yoltla/Miztli/Xiuhcoatl at LSVP UAM-Iztapalapa/DGTIC UNAM/CGSTIC CINVESTAV.
Funding
This work was supported by UNAM-PAPIIT IN218118, PIAPI1846-FESC-UNAM and LANCAD-UNAM-DGTIC-058.
Author information
Authors and Affiliations
Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Flores, R., Reyes-García, L.I., Rodríguez-Laguna, N. et al. Stability constants of Cu(II)/indomethacin mononuclear complexes in solution. Theor Chem Acc 137, 125 (2018). https://doi.org/10.1007/s00214-018-2315-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-018-2315-z