Abstract
Rationale
Multiple psychiatric disorders are associated with altered brain and serum levels of neuroactive steroids, including the endogenous GABAergic steroid, allopregnanolone. Clinically, chronic cocaine use was correlated with decreased levels of pregnenolone. Preclinically, the effect of acute cocaine on allopregnanolone levels in rodents has had mixed results, showing an increase or no change in allopregnanolone levels in some brain regions.
Objective
We hypothesized that cocaine acutely increases allopregnanolone levels, but repeated cocaine exposure decreases allopregnanolone levels compared to controls.
Methods
We performed two separate studies to determine how systemic administration of 15 mg/kg cocaine (1) acutely or (2) chronically alters brain (olfactory bulb, frontal cortex, dorsal striatum, and midbrain) and serum allopregnanolone levels in adult male and female Sprague-Dawley rats.
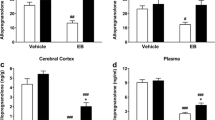
Results
Cocaine acutely increased allopregnanolone levels in the midbrain, but not in olfactory bulb, frontal cortex, or dorsal striatum. Repeated cocaine did not persistently (24 h later) alter allopregnanolone levels in any region in either sex. However, allopregnanolone levels varied by sex across brain regions. In the acute study, we found that females had significantly higher allopregnanolone levels in serum and olfactory bulb relative to males. In the repeated cocaine study, females had significantly higher allopregnanolone levels in olfactory bulb, frontal cortex, and serum. Finally, acute cocaine increased allopregnanolone levels in the frontal cortex of females in proestrus, relative to non-proestrus stages.
Conclusion
Collectively these results suggest that allopregnanolone levels vary across brain regions and by sex, which may play a part in differential responses to cocaine by sex.





Similar content being viewed by others
Data availability
The raw datasets used and/or analyzed during the current study will be made available upon reasonable request.
References
Aedo AR, Landgren BM, Diczfalusy E (1981) Studies on ovarian and adrenal steroids at different phases of the menstrual cycle: II. A comparative assessment of the circadian variation in steroid and lutropin levels during the follicular, periovulatory and luteal phases. Contraception 23(4):407–24. https://doi.org/10.1016/0010-7824(81)90030-5. PMID: 7273761
Anker JJ, Holtz NA, Zlebnik N, Carroll ME (2009) Effects of allopregnanolone on the reinstatement of cocaine-seeking behavior in male and female rats. Psychopharmacology 203(1):63–72
Barbaccia ML, Roscetti G, Trabucchi M, Mostallino MC, Concas A, Purdy RH, Biggio G (1996) Time-dependent changes in rat brain neuroactive steroid concentrations and GABAA receptor function after acute stress. Neuroendocrinology 63(2):166–72. https://doi.org/10.1159/000126953. PMID: 9053781
Bixo M, Andersson A, Winblad B, Purdy RH, Bäckström T (1997) Progesterone, 5alpha-pregnane-3,20-dione and 3alpha-hydroxy-5alpha-pregnane-20-one in specific regions of the human female brain in different endocrine states. Brain Res 764(1–2):173–8. https://doi.org/10.1016/s0006-8993(97)00455-1. PMID: 9295207
Boero G, Porcu P, Morrow AL (2019) Pleiotropic actions of allopregnanolone underlie therapeutic benefits in stress-related disease. Neurobiol Stress 12:100203. https://doi.org/10.1016/j.ynstr.2019.100203PMID: 31879693; PMCID: PMC6920111
Boero G, Tyler RE, O’Buckley TK, Balan I, Besheer J, Morrow AL (2022) (3α,5α)3-Hydroxypregnan-20-one (3α,5α-THP) regulation of the HPA Axis in the context of different stressors and sex. Biomolecules 12(8):1134. https://doi.org/10.3390/biom12081134PMID: 36009028; PMCID: PMC9406198
Boero G, Tyler RE, Todd CA, O’Buckley TK, Balan I, Besheer J, Morrow AL (2021) (3α,5α)3-hydroxypregnan-20-one (3α,5α-THP) regulation of hypothalamic and extrahypothalamic corticotropin releasing factor (CRF): sexual dimorphism and brain region specificity in Sprague Dawley rats. Neuropharmacology 186:108463 Epub 2021 Jan 16. PMID: 33460689; PMCID: PMC8010646
Caligioni CS (2009) Assessing reproductive status/stages in mice. Curr Protoc Neurosci Appendix 4:Appendix 4I. https://doi.org/10.1002/0471142301.nsa04is48. PMID: 19575469; PMCID: PMC2755182
Calipari ES, Juarez B, Morel C, Walker DM, Cahill ME, Ribeiro E, Roman-Ortiz C, Ramakrishnan C, Deisseroth K, Han MH, Nestler EJ (2017) Dopaminergic dynamics underlying sex-specific cocaine reward. Nat Commun 8:13877. https://doi.org/10.1038/ncomms13877PMID: 28072417; PMCID: PMC5234081
Castelli MP, Casti A, Casu A, Frau R, Bortolato M, Spiga S, Ennas MG (2013) Regional distribution of 5α-reductase type 2 in the adult rat brain: an immunohistochemical analysis. Psychoneuroendocrinology 38(2):281–293. https://doi.org/10.1016/j.psyneuen.2012.06.008Epub 2012 Jul 8. PMID: 22776423; PMCID: PMC3762250
CDC, National Center for Health Statistics (2022) U.S. Overdose deaths in 2021 increased half as much as in 2020 – but are still up 15% https://www.cdc.gov/nchs/pressroom/nchs_press_releases/2022/202205.htm. Accessed 3 February 2023
Cheng KC, Lee J, Khanna M, Qin KN (1994) Distribution and ontogeny of 3 alpha-hydroxysteroid dehydrogenase in the rat brain. J Steroid Biochem Mol Biol 50(1–2):85–9. https://doi.org/10.1016/0960-0760(94)90175-9. PMID: 8049137
Compagnone NA, Mellon SH (2000) Neurosteroids: biosynthesis and function of these novel neuromodulators. Front Neuroendocr 21(1):1–56
Diviccaro S, Cioffi L, Falvo E, Giatti S, Melcangi RC, Allopregnanolone (2022) An overview on its synthesis and effects. J Neuroendocrinol 34(2):e12996. https://doi.org/10.1111/jne.12996Epub 2021 Jun 29. PMID: 34189791; PMCID: PMC9285581
Dornellas APS, Macedo GC, McFarland MH, Gómez-A A, O’Buckley TK, Da Cunha C, Morrow AL, Robinson DL (2021) Allopregnanolone decreases evoked dopamine release differently in rats by sex and estrous stage. Front Pharmacol 11:608887. https://doi.org/10.3389/fphar.2020.608887PMID: 33519475; PMCID: PMC7840599
Ellinwood EH Jr, Balster RL (1974) Rating the behavioral effects of amphetamine. Eur J Pharmacol 28(1):35–41. https://doi.org/10.1016/0014-2999(74)90109-5. PMID: 4473346
Evans SM, Foltin RW (2010) Does the response to cocaine differ as a function of sex or hormonal status in human and non-human primates? Horm Behav 58(1):13–21. https://doi.org/10.1016/j.yhbeh.2009.08.010Epub 2009 Sep 4. PMID: 19733571; PMCID: PMC2883681
Finn DA, Sinnott RS, Ford MM, Long SL, Tanchuck MA, Phillips TJ (2004) Sex differences in the effect of ethanol injection and consumption on brain allopregnanolone levels in C57BL/6 mice. Neuroscience 123(4):813–9. https://doi.org/10.1016/j.neuroscience.2003.11.017. PMID: 14751275
Genazzani AR, Petraglia F, Bernardi F, Casarosa E, Salvestroni C, Tonetti A, Nappi RE, Luisi S, Palumbo M, Purdy RH, Luisi M (1998) Circulating levels of allopregnanolone in humans: gender, age, and endocrine influences. J Clin Endocrinol Metab 83(6):2099–103. https://doi.org/10.1210/jcem.83.6.4905. PMID: 9626145
Grobin AC, VanDoren MJ, Porrino LJ, Morrow AL (2005) Cortical 3 alpha-hydroxy-5 alpha-pregnan-20-one levels after acute administration of Delta 9-tetrahydrocannabinol, cocaine and morphine. Psychopharmacology 179(3):544–550. https://doi.org/10.1007/s00213-004-2084-3Epub 2004 Dec 24. PMID: 15619118
Handa RJ, Weiser MJ (2014) Gonadal steroid hormones and the hypothalamo–pituitary–adrenal axis. Front Neuroendocr 35(2):197–220. https://doi.org/10.1016/j.yfrne.2013.11.001
Hu M, Crombag HS, Robinson TE, Becker JB (2004) Biological basis of sex differences in the propensity to self-administer cocaine. Neuropsychopharmacology 29(1):81–5. https://doi.org/10.1038/sj.npp.1300301. PMID: 12955098
Johnson AR, Thibeault KC, Lopez AJ, Peck EG, Sands LP, Sanders CM, Kutlu MG, Calipari ES (2019) Cues play a critical role in estrous cycle-dependent enhancement of cocaine reinforcement. Neuropsychopharmacology 44(7):1189–1197. https://doi.org/10.1038/s41386-019-0320-0Epub 2019 Jan 23. PMID: 30728447; PMCID: PMC6785030
Kimball A, Dichtel LE, Nyer MB, Mischoulon D, Fisher LB, Cusin C, Dording CM, Trinh NH, Yeung A, Haines MS, Sung JC, Pinna G, Rasmusson AM, Carpenter LL, Fava M, Klibanski A, Miller KK (2020) The allopregnanolone to progesterone ratio across the menstrual cycle and in menopause. Psychoneuroendocrinology 112:104512 Epub 2019 Nov 14. PMID: 31780185; PMCID: PMC6935417
King TS, Schenken RS, Kang IS, Javors MA, Riehl RM (1990) Cocaine disrupts estrous cyclicity and alters the reproductive neuroendocrine axis in the rat. Neuroendocrinology 51(1):15–22. https://doi.org/10.1159/000125310. PMID: 2106083
Lynch WJ, Roth ME, Mickelberg JL, Carroll ME (2001) Role of estrogen in the acquisition of intravenously self-administered cocaine in female rats. Pharmacol Biochem Behav 68(4):641–6. https://doi.org/10.1016/s0091-3057(01)00455-5. PMID: 11526960
Martinez LA, Gross KS, Himmler BT, Emmitt NL, Peterson BM, Zlebnik NE, Foster Olive M, Carroll ME, Meisel RL, Mermelstein PG (2016) Estradiol facilitation of cocaine self-administration in female rats requires activation of mGluR5. eNeuro 3(5):ENEURO.0140–16.2016. https://doi.org/10.1523/ENEURO.0140-16.2016. PMID: 27822496; PMCID: PMC5079229
Mello NK, Negus SS, Knudson IM, Kelly M, Mendelson JH (2008) Effects of estradiol on cocaine self-administration and cocaine discrimination by female rhesus monkeys. Neuropsychopharmacology 33(4):783–795
Mesen TB, Young SL (2015) Progesterone and the luteal phase: a requisite to reproduction. Obstet Gynecol Clin North Am 42(1):135–151. https://doi.org/10.1016/j.ogc.2014.10.003Epub 2015 Jan 5. PMID: 25681845; PMCID: PMC4436586
Milivojevic V, Covault J, Angarita GA, Siedlarz K, Sinha R (2019) Neuroactive steroid levels and cocaine use chronicity in men and women with cocaine use disorder receiving progesterone or placebo. Am J Addict 28:16–21
Milivojevic V, Fox HC, Sofuoglu M, Covault J, Sinha R (2016) Effects of progesterone stimulated allopregnanolone on craving and stress response in cocaine dependent men and women. Psychoneuroendocrinology 65:44–53
Paxinos G, Watson C (2006) The rat brain in stereotaxic coordinates. Academic Press, London
Porcu P, Barron AM, Frye CA, Walf AA, Yang SY, He XY, Morrow AL, Panzica GC, Melcangi RC (2016) Neurosteroidogenesis today: novel targets for neuroactive steroid synthesis and action and their relevance for translational research. J Neuroendocrinol 28:12351
Porcu P, Rogers LS, Morrow AL, Grant KA (2006) Plasma pregnenolone levels in cynomolgus monkeys following pharmacological challenges of the hypothalamic-pituitary-adrenal axis. Pharmacol Biochem Behav 84(4):618–627. https://doi.org/10.1016/j.pbb.2006.05.004Epub 2006 Jun 21. PMID: 16790266
Quinones-Jenab V, Minerly AC, Niyomchia T, Akahvan A, Jenab S, Frye C (2008) Progesterone and allopregnanolone are induced by cocaine in serum and brain tissues of male and female rats. Pharmacol Biochem Behav 89(3):292–297. https://doi.org/10.1016/j.pbb.2007.12.024Epub 2008 Jan 7. PMID: 18255131
Rougé-Pont F, Mayo W, Marinelli M, Gingras M, Le Moal M, Piazza PV (2002) The neurosteroid allopregnanolone increases dopamine release and dopaminergic response to morphine in the rat nucleus accumbens. Eur J Neurosci 16(1):169–73. https://doi.org/10.1046/j.1460-9568.2002.02084.x. PMID: 12153544
Rustichelli C, Bellei E, Bergamini S, Monari E, Baraldi C, Castro FL, Tomasi A, Ferrari A (2020) Serum levels of allopregnanolone, progesterone and testosterone in menstrually-related and postmenopausal migraine: a cross-sectional study. Cephalalgia 40(12):1355–1362 Epub 2020 Jun 26. PMID: 32588652; PMCID: PMC7575305
Sinha R, Fox H, Hong KI, Sofuoglu M, Morgan PT, Bergquist KT (2007) Sex steroid hormones, stress response, and drug craving in cocaine-dependent women: Implications for relapse susceptibility. Exp Clin Psychopharmacol 15(5):445–452. PMID: 17924778. https://doi.org/10.1037/1064-1297.15.5.445
Smith MS, Fox SR, Chatterton RT (1989) Role of proestrous progesterone secretion in suppressing basal pulsatile LH secretion during estrus of the estrous cycle. Neuroendocrinology 50(3):308–314
Smith MS, Freeman ME, Neill JD (1975) The control of progesterone secretion during the estrous cycle and early pseudopregnancy in the rat: prolactin, gonadotropin and steroid levels associated with rescue of the corpus luteum of pseudopregnancy. Endocrinology 96:219–226
Substance Abuse and Mental Health Services Administration (2021) Key substance use and mental health indicators in the United States: results from the 2020 national survey on drug use and health (HHS Publication No. PEP21-07-01-003, NSDUH Series H-56). Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration, Rockville, MD. Retrieved from https://www.samhsa.gov/data/
Sze Y, Gill AC, Brunton PJ (2018) Sex-dependent changes in neuroactive steroid concentrations in the rat brain following acute swim stress. J Neuroendocrinol 30(11):e12644. https://doi.org/10.1111/jne.12644Epub 2018 Oct 7. PMID: 30194779; PMCID: PMC6221110
Thanos PK, Subrize M, Lui W, Puca Z, Ananth M, Michaelides M, Wang GJ, Volkow ND (2011) D-cycloserine facilitates extinction of cocaine self-administration in C57 mice. Synapse 65(10):1099–1105. https://doi.org/10.1002/syn.20944Epub 2011 May 16. PMID: 21584863; PMCID: PMC3192019
Walker Q, Cabassa J, Kaplan K et al (2001) Sex differences in cocaine-stimulated motor behavior: disparate effects of gonadectomy. Neuropsychopharmacol 25:118–130. https://doi.org/10.1016/S0893-133X(00)00248-7
Witten IB, Lin SC, Brodsky M, Prakash R, Diester I, Anikeeva P, Gradinaru V, Ramakrishnan C, Deisseroth K (2010) Cholinergic interneurons control local circuit activity and cocaine conditioning. Sci (New York N Y) 330(6011):1677–1681. https://doi.org/10.1126/science.1193771
Zhang D, Yang S, Yang C, Jin G, Zhen X (2008) Estrogen regulates responses of dopamine neurons in the ventral tegmental area to cocaine. Psychopharmacology 199(4):625–635. https://doi.org/10.1007/s00213-008-1188-6Epub 2008 Jun 1. PMID: 18516717
Acknowledgements
The authors would like to thank Abigail Edmonds for her assistance with experiments and data analysis, and Dr. Victoria Macht, Dr. Alexander Gómez-A, and Dr. Chris Weisen for their assistance with statistical analysis and interpretation.
Funding
This research was funded by a Foundation of Hope (Raleigh, NC, United States) research award and the Bowles Center for Alcohol Studies. MHM was supported on “UNC PREP in the Biomedical Sciences” (NIH R25 GM089569), the UNC Neurobiology Predoctoral Training Grant (NINDS T32 NS007431), and a National Research Service Award (NIDA F31 DA054781). MMFM was supported on a Predoctoral CAPES Foundation fellowship - Coordination of Improvement of Higher Education Personnel (Brazil). CCC was supported on “Molecular and Cellular Alcohol Research Training” (NIAAA T32 AA007573).
Author information
Authors and Affiliations
Contributions
MHM, MMFM, DLR: conception and design of work; MHM, MMFM, GMS, KCM, TKO: data acquisition and analysis; MHM, MMFM, TKO, GB, CCC, ALM, DLR: interpretation of data; MHM and DLR: drafted work; All authors edited, read, and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This animal study was reviewed and approved by the Institutional Animal Care and Use Committee of University of North Carolina at Chapel Hill.
Conflict of interest
The authors have no conflict of interest or competing interests to declare.
Financial interests
The authors have no financial interests to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
McFarland, M.H., Machado, M.M.F., Sansbury, G.M. et al. Acute, but not repeated, cocaine exposure alters allopregnanolone levels in the midbrain of male and female rats. Psychopharmacology 241, 1011–1025 (2024). https://doi.org/10.1007/s00213-024-06534-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-024-06534-8