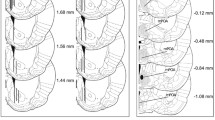
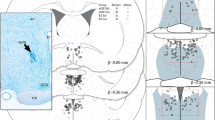
Abstract
Rationale
Sex differences in cocaine abuse have been well documented. However, the underlying mechanism remains unclear.
Objectives
To explore the potential role of ovarian hormones in the regulation of dopamine (DA) neuron firing activity in ventral tegmental area (VTA) induced by acute cocaine in intact female or ovariectomized (OVX) rats.
Results
The basal firing activity of VTA DA neurons was changed in a manner phase-locked to the estrous cycle: being highest in estrus and lowest in proestrus. Acute cocaine produced greater inhibition (P < 0.05) on the firing of VTA DA neurons during proestrus than during estrus. The inhibitory effect was completely blocked by OVX and restored by replacement of 17-β-estradiol or, to a less extent, by replacement of progesterone. In addition, we also detected female hormone-associated changes in slow oscillation in VTA DA neurons. The results indicate that ovarian hormones, particularly estrogen, not only synergize with the inhibitory effect of cocaine on VTA DA neuron activity but also play an essential role in maintaining the sensitivity of DA neurons to cocaine-mediated inhibition on firing. Moreover, pretreatment of estrogen receptor (ER) antagonist raloxifene or a selective ERα antagonist Y134 largely attenuated the cocaine-inhibited DA neuron firing. We also found that cocaine-induced locomotor activity was estrous cycle dependant; 17-β-estradiol but not progesterone replacement restored the cocaine-induced locomotor activity in OVX rats.
Conclusion
The present results demonstrated that ovarian hormones, particularly estrogen, produce profound effect on VTA DA neuron activity, which, in turn, may contribute to the sex differences in response to psychostimulants.








Similar content being viewed by others
References
Bazzett TJ, Becker H (1994) Sex differences in the rapid and acute effects of estrogen on striatal D2 dopamine receptor binding. Brain Res 637:163–172
Becker JB (1990) Estrogen rapidly potentiates amphetamine-induced striatal dopamine release and rotational behavior during microdialysis. Neurosci Lett 118:169–171
Becker JB (1999) Gender differences in dopaminergic function in striatum and nucleus accumbens. Pharmacol Biochem Behav 64:803–812
Becker JB, Ramirez VD (1981) Experimental studies on the development of sex differences in the release of dopamine from striatal tissue fragments in vitro. Neuroendocrinology 32:168–173
Becker JB, Rudick CN (1999) Rapid effects of estrogen or progesterone on the amphetamine-induced increase in striatal dopamine are enhanced by estrogen priming: a microdialysis study. Pharmacol Biochem Behav 64:53–57
Becker JB, Hu M (2008) Sex differences in drug abuse. Front Neuroendocrinol 29:36–47
Bosse R, Rivest R, Di Paolo T (1997) Ovariectomy and estradiol treatment affect the dopamine transporter and its gene expression in the rat brain. Brain Res Mol Brain Res 46:343–346
Carroll ME, Lynch WJ, Roth ME, Morgan AD, Cosgrove KP (2004) Sex and estrogen influence drug abuse. Tips 25:273–279
Castner SA, Xiao L, Becker JB (1993) Sex differences in striatal dopamine: in vivo microdialysis and behavioral studies. Brain Res 610:127–134
Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O’Brien CP (1999) Limbic activation during cue-induced cocaine craving. Am J Psychiatry 156:11–18
Chiodo LA (1988) Dopamine-containing neurons in the mammalian central nervous system: electrophysiology and pharmacology. Neurosci and Biobehav Rev 12:49–91
Chiodo LA, Bunney BS (1985) Possible mechanisms by which repeated clozapine administration differentially affects the activity of two subpopulations of midbrain dopamine neurons. J Neurosci 5:2539–2544
Dazzi L, Seu E, Cherchi G, Barbieri PP, Matzeu A, Biggio G (2007) Estrous cycle-dependent changes in basal and ethanol-induced activity of cortical dopaminergic neurons in the rat. Neuropsychopharmacol 32:892–901
Di Paolo T (1994) Modulation of brain dopamine transmission by sex steroids. Rev Neurosci 5:27–41
Dluzen DE, Ramirez VD (1987) Intermittent infusion of progesterone potentiates whereas continuous infusion reduces amphetamine-stimulated dopamine release from ovariectomized estrogen-primed rat striatal fragments superfused in vitro. Brain Res. 406:1–9
Gao M, Liu CL, Yang S, Jin GZ, Bunney BS, Shi WX (2007) Functional coupling between the prefrontal cortex and dopamine neurons in the ventral tegmental area. J Neurosci 27:5414–5421
Goldman-Rakic PS (1987) Circuitry of the frontal association cortex and its relevance to dementia. Arch Gerontol Geriatr 6:299–309
Grace AA, Bunney BS (1983a) Intracellular and extracellular electrophysiology of nigral dopaminergic neurons—2. Action potential generating mechanisms and morphological correlates. Neurosci 10:317–331
Grace AA, Bunney BS (1983b) Intracellular and extracellular electrophysiology of nigral dopaminergic neurons—1. Identification and characterization. Neurosci 10:301–315
Grace AA, Bunney BS (1983c) Intracellular and extracellular electrophysiology of nigral dopaminergic neurons—3. Evidence for electrotonic coupling. Neurosci 10:333–348
Grace AA, Bunney BS (1986) Induction of depolarization block in midbrain dopamine neurons by repeated administration of haloperidol: analysis using in vivo intracellular recording. J Pharmacol Exp Ther 238:1092–1100
Hu M, Becker JB (2003) Effects of sex and estrogen on behavioral sensitization to cocaine in rats. J Neurosci 23:693–699
Jentsch JD, Redmond DE Jr., Elsworth JD, Taylor JR, Youngren KD, Roth RH (1997) Enduring cognitive deficits and cortical dopamine dysfunction in monkeys after long-term administration of phencyclidine. Science 277:953–955
Lammers CH, D’Souza U, Qin ZH, Lee SH, Yajima S, Mouradian MM (1999) Regulation of striatal dopamine receptors by estrogen. Synapse 34:222–227
Landry M, Levesque D, Di Paolo T (2002) Estrogenic properties of raloxifene, but not tamoxifen, on D2 and D3 dopamine receptors in the rat forebrain. Neuroendocrinology 76:214–222
Leranth C, Roth RH, Elsworth JD, Naftolin F, Horvath TL, Redmond DE Jr. (2000) Estrogen is essential for maintaining nigrostriatal dopamine neurons in primates: implications for Parkinson’s disease and memory. J Neurosci 20:8604–8609
Lynch WJ, Roth ME, Carroll ME (2002) Biological basis of sex differences in drug abuse: preclinical and clinical studies. Psychopharmacol (Berl) 164:121–137
McEwen B (2002) Estrogen actions throughout the brain. Recent Prog Horm Res 57:357–384
McEwen BS, Alves SE (1999) Estrogen actions in the central nervous system. Endocr Rev 20:279–307
Morissette M, Di Paolo T (1993a) Effect of chronic estradiol and progesterone treatments of ovariectomized rats on brain dopamine uptake sites. J Neurochem 60:1876–1883
Morissette M, Di Paolo T (1993b) Sex and estrous cycle variations of rat striatal dopamine uptake sites. Neuroendocrinology 58:16–22
Ning M, Zhou C, Weng J, Zhang S, Chen D, Yang C, Wang H, Ren J, Zhou L, Jin C, Wang MW (2007) Biological activities of a novel selective oestrogen receptor modulator derived from raloxifene (Y134). Br J Pharmacol 150:19–28
Niyomchai T, Russo SJ, Festa ED, Akhavan A, Jenab S, Quinones-Jenab V (2005) Progesterone inhibits behavioral responses and estrogen increases corticosterone levels after acute cocaine administration. Pharmacol Biochem and Behav 80:603–610
O’Donnell P (2003) Dopamine gating of forebrain neural ensembles. Eur J Neurosci 17:429–435
Ohtani H, Nomoto M, Douchi T (2001) Chronic estrogen treatment replaces striatal dopaminergic function in ovariectomized rats. Brain Res 900:163–168
Quinones-Jenab V (2006) Why are women from Venus and men from Mars when they abuse cocaine? Brain Res 1126:200–203
Quinones-Jenab V, Perrotti LI, Mc Monagle J, Ho A, Kreek MJ (2000) Ovarian hormone replacement affects cocaine-induced behaviors in ovariectomized female rats. Pharmacol Biochem and Behav 67:417–422
Ritz MC, Lamb RJ, Goldberg SR, Kuhar MJ (1987) Cocaine receptors on dopamine transporters are related to self-administration of cocaine. Science 237:1219–1223
Roth ME, Cosgrove KP, Carroll ME (2004) Sex differences in the vulnerability to drug abuse: a review of preclinical studies. Neurosci and Biobehav Rev 28:533–546
Sell SL, Scalzitti JM, Thomas ML, Cunningham KA (2000) Influence of ovarian hormones and estrous cycle on the behavioral response to cocaine in female rats. J Pharmacol Exp Ther 293:879–886
Sell SL, Thomas ML, Cunningham KA (2002) Influence of estrous cycle and estradiol on behavioral sensitization to cocaine in female rats. Drug Alcohol Depend 67:281–290
Shi WX (2005) Slow oscillatory firing: a major firing pattern of dopamine neurons in the ventral tegmental area. J Neurophysiol 94:3516–3522
Takuma K, Matsuo A, Himeno Y, Hoshina Y, Ohno Y, Funatsu Y, Arai S, Kamei H, Mizoguchi H, Nagai T, Koike K, Inoue M, Yamada K (2007) 17[beta]-estradiol attenuates hippocampal neuronal loss and cognitive dysfunction induced by chronic restraint stress in ovariectomized rats. Neurosci 146:60–68
Thompson TL, Moss RL (1997) Modulation of mesolimbic dopaminergic activity over the rat estrous cycle. Neurosci Lett 229:145–148
Torres-Hernandez AR, Gonzalez-Vegas JA (2005) Effects of 17beta-estradiol on the spontaneous activity of substantia nigra neurons: evidence for a non-genomic mechanism. Brain Res 1049:1–7
Tzschentke TM (2001) Pharmacology and behavioral pharmacology of the mesocortical dopamine system. Prog Neurobiol 63:241–320
Tzschentke TM, Schmidt WJ (2000) Functional relationship among medial prefrontal cortex, nucleus accumbens, and ventral tegmental area in locomotion and reward. Crit Rev Neurobiol 14:131–142
Walker QD, Cabassa J, Kaplan KA, Li ST, Haroon J, Spohr HA, Kuhn CM (2001) Sex differences in cocaine-stimulated motor behavior: disparate effects of gonadectomy. Neuropsychopharmacol 25:118–130
Xiao L, Becker JB (1994) Quantitative microdialysis determination of extracellular striatal dopamine concentration in male and female rats: effects of estrous cycle and gonadectomy. Neurosci Lett 180:155–158
Zhang D, Yang S, Jin G-Z, Bunney BS, Shi W-X (2007) Oscillatiory firing of dopamine neurons: differences between cells in the substantia Nigra and Ventral Tegmental area. Synapse 62:169–175
Zhen X, Goswami S, Abdali SA, Frankfurt M, Friedman E (2007) Estrogen-modulated frontal cortical CaMKII activity and behavioral supersensitization induced by prolonged cocaine treatment in female rats. Psychopharmacol (Berl) 191:323–331
Zhou W, Cunningham KA, Thomas ML (2002) Estrogen regulation of gene expression in the brain: a possible mechanism altering the response to psychostimulants in female rats. Brain Res Mol Brain Res 100:75–83
Zhu ZT, Fu Y, Hu GY, Jin GZ (2000) Modulation of medical prefrontal cortical D1 receptors on the excitatory firing activity of nucleus accumbens neurons elicited by (−)-Stepholidine. Life Sci 67:1265–1274
Acknowledgments
This work was supported by the National Basic Research plan of the Ministry of Science and Technology (2007AA02z163; 2007CB935804), a Pujiang-plan grant from the Shanghai Commission of Science and Technology (07pj14104), and a grant Natural Science Foundation of China (30770662).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, D., Yang, S., Yang, C. et al. Estrogen regulates responses of dopamine neurons in the ventral tegmental area to cocaine. Psychopharmacology 199, 625–635 (2008). https://doi.org/10.1007/s00213-008-1188-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-008-1188-6