Abstract
Hydrogen sulfide (H2S) is a gasotransmitter that has been studied for its potential therapeutic effects, including its role in the pathophysiology and treatment of stroke. This systematic review and meta-analysis aimed to determine the sufficiency of overall preclinical evidence to guide the initiation of clinical stroke trials with H2S and provide tailored recommendations for their design. PubMed, Web of Science, Scopus, EMBASE, and MEDLINE were searched for studies evaluating the effect of any H2S donor on in vivo animal models of regional ischemic stroke, and 34 publications were identified. Pooling of the effect sizes using the random-effect model revealed that H2S decreased the infarct area by 34.5% (95% confidence interval (CI) 28.2–40.8%, p < 0.0001), with substantial variability among the studies (I2 = 89.8%). H2S also caused a 37.9% reduction in the neurological deficit score (95% CI 29.0–46.8%, p < 0.0001, I2 = 63.8%) and in the brain water content (3.2%, 95% CI 1.4–4.9%, p = 0.0014, I2 = 94.6%). Overall, the studies had a high risk of bias and low quality of evidence (median quality score 5/15, interquartile range 4–9). The majority of the included studies had a “high” or “unclear” risk of bias, and none of the studies overall had a “low” risk. In conclusion, H2S significantly improves structural and functional outcomes in in vivo animal models of ischemic stroke. However, the level of evidence from preclinical studies is not sufficient to proceed to clinical trials due to the low external validity, high risk of bias, and variable design of existing animal studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Stroke is the second most prevalent cause of death and acquired adult disability worldwide (Saini et al. 2021). Despite a decline in mortality rates, the overall burden of stroke is on the rise.
Approximately 80% of all strokes are ischemic, resulting from the blockage of cerebral arteries (Virani et al. 2020). Ischemic stroke treatment has traditionally focused on reperfusion techniques such as intravenous thrombolysis with tissue plasminogen activator (tPA) and endovascular therapy, which are effective but limited by a narrow therapeutic window and risk of hemorrhage (Powers et al. 2018). Other treatment options include stem cell therapy, which promotes neurogenesis and reduces inflammation; natural products, peptides, and other pharmacological agents which decrease excitotoxicity, oxidative stress, apoptosis, and neuroinflammation; and nanomedicine, which targets drug delivery to the brain to improve outcomes (Lyden 2021; Jia et al. 2024). Although these emerging treatment options showed promising results in animal studies, translational challenges, including low study quality and publication bias in preclinical studies, have hindered their clinical application (Macleod et al. 2008; Sena et al. 2010). Consequently, it is advisable to conduct a well-designed systematic review and meta-analysis of preclinical stroke studies before embarking on clinical trials to provide nonbiased summaries of the available evidence (Bath et al. 2009; Dirnagl and Macleod 2009).
Hydrogen sulfide (H2S) has emerged as a candidate agent, and its effectiveness has been evaluated in various experimental animal models of ischemic stroke, yielding conflicting results (Gopalakrishnan et al. 2019; Jia et al. 2019; Ding et al. 2023). H2S functions as an endogenously produced gasotransmitter and serves as a vital signaling molecule at low concentrations, playing a significant role in vasodilation and blood pressure regulation (Kimura 2021). Growing evidence suggests that exogenous H2S donors protect organs, including the brain, from ischemia-reperfusion injury by mitigating inflammation, oxidative stress, mitochondrial damage, and apoptosis (Gopalakrishnan et al. 2019; Jia et al. 2019; Ding et al. 2023; Emre Aydıngöz et al. 2023). H2S therapy offers significant advantages over tPA by reducing hemorrhagic complications for ischemic stroke treatment (Liu et al. 2016; Jia et al. 2019). Combining H2S with tPA can improve safety and efficacy, suggesting a promising approach for enhancing stroke management (Liu et al. 2016).
Despite the extensive exploration of the beneficial effects of H2S against ischemic stroke in individual animal studies, no clinical trials have verified its efficacy in human settings. Furthermore, although many preclinical studies and narrative reviews have shown that H2S may be effective for treating stroke (Dou et al. 2016; Chan and Wong 2017; Gopalakrishnan et al. 2019; Narne et al. 2019; Ding et al. 2023), a systematic review of animal studies and quantification of the available data by meta-analysis have not yet been performed. Given the persistent translational challenges in preclinical stroke studies, a thorough systematic review and meta-analysis of existing evidence on the effectiveness of H2S against ischemic stroke is crucial.
This systematic review and meta-analysis aimed to determine the sufficiency of overall preclinical evidence to guide the initiation of clinical stroke trials with H2S and provide tailored recommendations for their design.
The research question is “How effective is H2S compared with no treatment on structural and functional outcomes in in vivo animal models of ischemic stroke?”
Methods
Study protocol overview
This systematic review and meta-analysis was conducted following a registered PROSPERO protocol (CRD42023380938, date: 10/01/2023). No amendments were made to the study protocol afterward. The guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (Moher et al. 2009) and recommendations for the systematic review and meta-analysis of animal studies (Vesterinen et al. 2014; Soliman et al. 2020) were followed. The PRISMA checklist is presented in Supplement 1.
Literature search
The inclusion criterion was an in vivo animal model of regional ischemic stroke treated with any H2S donor. The exclusion criteria included in vitro, ex vivo, and in silico studies; observational studies; experimental studies lacking a control group; reviews; conference presentations; studies on human subjects; investigations involving interventions increasing endogenous H2S levels; and studies lacking crucial information for meta-analysis. There were no language or date restrictions.
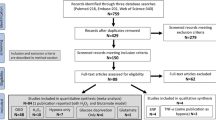
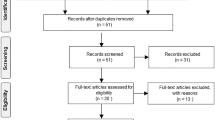
The PubMed, Web of Science, Scopus, EMBASE, and MEDLINE databases were searched using the specific criteria detailed in Supplement 2. Animal filters devised by van der Mierden et al. (2022) and de Vries et al. (2014) were applied to the PubMed and EMBASE databases. The Polyglot Search Translator (Clark et al. 2020) was utilized to translate the search strategies across databases. The initial search was performed on February 23, 2023, with an update on August 1, 2023. Duplicates were removed, and the title/abstract was prescreened using the SyRF screening application (http://app.syrf.org.uk/) (Bahor et al. 2021). Prescreening and full-text screening were performed by two independent reviewers, with discrepancies resolved through discussion with a third reviewer.
Data extraction and effect measures
The key study characteristics extracted were experimental group, number of animals per group, species, sex, age and weight of the animals, comorbidity status, anesthetic agent, ischemic model, cotreatment, H2S donor, dosage, administration time, and route. For the effect measures, data on infarct volume (primary outcome), neurological deficit score, and brain edema were extracted. Mechanistic outcome measures involving biomarkers are reported in five or more studies. In cases where outcomes were reported at different time points for the same group of animals, the last time point measurement was extracted.
For all outcome measures, published continuous data (mean) and variance (standard deviation/standard error of the mean) were extracted without unit conversion. In cases where the variance was given as the standard error of the mean, the standard deviation was estimated on the basis of the sample size. The data presented graphically were extracted using WebPlotDigitizer (version 4.5, August 2021, Pacifica, California, USA). For one study, the standard deviation for infarct volume was obtained from the author of the original paper (Genc et al. 2023). If it was not possible to obtain the necessary effect measures, the paper was not included in the meta-analysis. Two reviewers independently performed the data extraction using the SyRF application. In cases of < 10% difference between extracted data, an average of the two values was taken; discrepancies > 10% were resolved through discussion with a third reviewer. The extracted raw data are presented in Supplement 3.
Meta-analysis
A meta-analysis was conducted on the full texts of a predefined number of studies (at least 10) with similar study designs, comparing the effects of any H2S donor with no treatment on structural and functional outcomes in in vivo animal models of ischemic stroke. All analyses were performed using the “metafor” package (Viechtbauer 2010) in R 4.2.1 (R Foundation for Statistical Computing, Vienna, Austria; https://www.r-project.org/) and guided by the online version “Doing Meta-Analysis with R: A Hands-On Guide” (Harrer et al. 2021). The R codes are presented in Supplement 4.
Pooling of data
The data were pooled for the outcome measures that were reported in six or more studies. Pooling of effect sizes was accomplished through the calculation of the normalized mean difference (NMD) for infarct area, neurological deficit score, and brain edema, considering that the data were on a ratio scale. For other outcome measures, Hedges’ g (bias-corrected standardized mean difference (SMD)) served as the effect size. Random-effect models with a restricted maximum likelihood (RELM) estimate of between-study variance were employed to obtain pooled effect sizes for each outcome. The 95% confidence interval (CI) surrounding the pooled effect was computed using Knapp-Hartung adjustments (Knapp and Hartung 2003). Forest plots were generated to summarize the meta-analysis results. The sensitivity of the analysis was assessed by repeating it with a fixed-effect model and performing the leave-one-out test.
Assessment of heterogeneity
The between-study variance was estimated by calculating Tau2, Q (χ2), and its significance level. The percentage of residual variation attributable to between-study heterogeneity was expressed as I2. Meta-regression (subgroup) analysis was conducted to investigate the impact of various study characteristics on the treatment effect, including the type, dose, and application time of H2S donors; duration and mode of ischemia (temporary vs. permanent); age and species of animals; outcome assessment time; and risk of bias measures. The number of subgroups was minimized, defined according to the cutoff points identified by a data-driven approach (i.e., median), as suggested by Wang et al. (2021). Meta-regression analysis was performed on the pooled infarct volume data, the outcome measure reported by most of the studies.
Assessment of publication bias
Publication bias was assessed using small-study effect methods involving visual inspection of the funnel plot and quantification of asymmetry with Egger’s regression test. The trim-and-fill method was applied to address funnel plot asymmetry and obtain a corrected effect estimate. To avoid false-positive results, NMD was used as the effect size measure in the funnel plot, as suggested by Zwetsloot et al. (2017).
Assessment of quality and risk of bias
The quality of evidence for each study was evaluated using the CAMARADES study quality checklist (Sena et al. 2007), modified by McCann et al. (2016), to incorporate relevant items from the updated STAIR criteria (Fisher et al. 2009). The risk of bias was categorized according to the SYRCLE risk of bias tool (Hooijmans et al. 2014), and the risk of bias plot was generated using the web-based application robvis (https://mcguinlu.shinyapps.io/robvis/) (McGuinness and Higgins 2021). Two reviewers independently assessed study quality and risk of bias, with discrepancies resolved through discussion with a third reviewer.
The significance level was set at p < 0.05 throughout the analysis.
Results
Characteristics of the selected studies
Thirty-four publications meeting the study selection criteria were identified through a systematic review of the literature (Fig. 1). Seven of these studies were conducted in mice, and the remaining 27 were conducted in rats; all of these studies used a middle cerebral artery occlusion model to induce transient stroke. Ischemia was induced for a wide range of durations, from 1 to 24 h. With the exception of three publications where the sex of the animal was not specified, all studies used male animals. Only three studies were carried out on aged animals, and none of the studies used animals with comorbidities. Notably, most studies (26/34) used NaHS as the H2S donor, mostly by intraperitoneal administration, with varying doses (1–40 mg/kg) and times of administration (before, during, or after ischemia). The basic characteristics of the included studies and published measures are summarized in Supplement 5.
Pooled effect measures
Infarct area
The infarct area of 408 H2S-treated animals versus 232 control animals from 54 comparisons was reported in 31 studies—mm3 in 8 studies and the percentage of brain area in the remaining 23 studies. Pooling the NMDs using a random-effect model revealed that H2S decreased the infarct area by 34.5% (95% CI 28.2–40.8%, p < 0.0001) (Fig. 2). The fixed-effect model also revealed a large and significant effect of H2S (36.9% decrease in the infarct area 95% CI 35.2–38.6%, p < 0.01), indicating the sensitivity of the random-effect model to the variability between studies. In the leave-one-out analyses, the pooled estimate ranged between 33.7 and 35.9% when each of the studies was removed from the model and the analysis was repeated, indicating that the results of the meta-analysis were relatively stable and not strongly influenced by any single study (Supplement 6).
Heterogeneity analysis indicated substantial variability among the studies, with a χ2 (heterogeneity statistics) of 520.9 (df = 53, p < 0.0001) and an I2 of 89.8%, suggesting that approximately 90% of the total variability in effect sizes is due to true differences between studies rather than sampling error (Fig. 2). In the leave-one-out analysis, the iteration of the model by excluding each study caused the heterogeneity to vary between 88.3 and 90.0%, confirming that the sources of variability among studies were not primarily driven by one particular study (Supplement 6).
Neurological deficit score
The neurological deficits of the animals were assessed in 20 studies by various scales, including the Longa, Garcia, mNSS, Bederson, and Philips scoring systems. The most preferred scale among these was the Longa scoring system, which was employed in 161 H2S-treated animals versus 83 control animals in 15 comparisons from 9 studies. High scores indicate severe neurological deficits on the five-point (0 to 4) Longa scale (Longa et al., 1989). Overall, H2S caused a 37.9% reduction in the Longa score (95% CI 29.0–46.8%, p < 0.0001), with moderate variability between studies (I2 = 63.8%, χ2 = 38.6, df = 14, p = 0.0004) (Fig. 3).
Brain water content
The brain water content of 99 H2S-treated animals versus 62 control animals was reported in 9 studies as [(ischemic hemisphere wet weight − dry weight)/wet weight] × 100%. Compared to the control group, H2S caused a small but significant reduction in brain water content (3.2%, 95% CI 1.4–4.9%, p = 0.0014), with very high heterogeneity (I2 = 94.6%, χ2 = 277.1, df = 15, p < 0.0001) (Fig. 3).
Biochemical markers for apoptosis and inflammation
The levels of TUNEL and caspase-3, which are markers of apoptosis, and TNF-alpha and IL-1beta, which are proinflammatory cytokines, were reported in at least six studies. When the data were pooled using the random-effect model, H2S significantly decreased only TUNEL (Hedges’ g −2.0, 95% CI −3.0; −0.9, p = 0.0010) but had no significant effect on the remaining variables (Hedges’ g −2.1 [−4.4; 0.3], p = 0.0815 for caspase-3; −0.9 [−1.9; 0.1], p = 0.0670 for IL-1beta; −1.1 [−2.4; 0.2], p = 0.0797 for TNF-alpha) (Fig. 4). This finding may suggest that the inhibitory effect of H2S on caspase-independent apoptotic pathways or the early/intermediate stage of apoptosis, rather than its anti-inflammatory activity, plays a role in its beneficial effect on the experimental stroke model.
Subgroup analysis
The results of subgroup analyses are reported in Table 1, which shows the estimated effect in each subgroup, the p value of the test for subgroup differences, residual heterogeneity, and the amount of heterogeneity accounting for that study variable. As shown in Table 1, animal species; blinded outcome assessment; controlling temperature; the STAIR score of the study; duration of ischemia; measurement unit for infarct area, type, dose, and application time of H2S; and time of outcome measure assessment did not have a significant effect on the observed heterogeneity between studies. However, although the difference was slightly greater than the conventional threshold (p = 0.0551), it is remarkable that H2S donors other than NaHS induced a much greater reduction in infarct volume than did NaHS donors (40.0% vs. 27.9%). The pooled effect of each H2S donor is shown in a forest plot in Supplement 7. An unexpected finding in the subgroup analysis was that randomization significantly increased the effect size (p = 0.01). Unexpectedly, H2S was found to cause a significantly greater reduction in the infarct area in randomized experiments, accounting for 13.3% of the heterogeneity.
Publication bias
The visual inspection of the funnel plot and Egger’s regression test (t = −0.89, df = 52, p = 0.3784) revealed no evidence of funnel plot asymmetry (Fig. 5). Parallel to this finding, the trim-and-fill method revealed no missing studies and did not adjust the model.
A Funnel plot representing the relationship between effect size and standard error (precision) for the studies included in the meta-analysis. The y-axis displays the standard error of the effect size, inversely proportional to study precision, while the x-axis represents the effect size estimate, which is the normalized mean difference (NMD) as a percent reduction in infarct volume. Each point on the plot corresponds to an individual study included in the meta-analysis. The diagonal line in the center represents the overall effect estimate. B A scatter plot of effect size against precision with the Egger regression line overlaid (t = −0.89, df = 52, p = 0.3784)
Study quality and risk of bias
According to the CAMARADES study quality checklist modified to include relevant items from the updated STAIR criteria, studies had low quality and high risk of bias (median score 5/15, interquartile range 4–9). Randomization of group allocation, blinded induction of ischemia, and blinded assessment of outcome—factors whose absence created the highest risk of bias—were reported in only 65%, 3%, and 50% of the studies, respectively (Table 2). Categorization of risk of bias by using the SYRCLE risk of bias tool revealed that the majority of the included studies had a “high” or “unclear” risk of bias, and none of the studies overall had a “low” risk (Fig. 6).
Discussion
To our knowledge, this is the first meta-analysis of preclinical studies evaluating the effect of H2S in an experimental stroke model. The analysis revealed that compared with no treatment, H2S significantly improved structural and functional outcomes in in vivo animal models of ischemic stroke. However, when considered as a whole, the level of evidence from preclinical studies is not sufficient to proceed to clinical trials due to the low external validity, high risk of bias, and variable design of existing animal studies.
Neuroprotective effects of H2S
Among the studies included in the meta-analysis, infarct volume was reported in 31 (91.2%), whereas neurological deficit scores were reported in only 20 (58.8%). Although these percentages are similar to those found in preclinical stroke studies in the literature, nearly all clinical stroke trials have reported functional outcome as the primary endpoint, and fewer than 15% of clinical trials have reported infarct volume (Schmidt-Pogoda et al. 2020). This factor may reduce the clinical translation of animal studies evaluating the effect of H2S on stroke. A meta-analysis of these studies indicated that H2S is very effective against stroke, decreasing the infarct area by 34.5% (95% CI 28.2–40.8%, p < 0.0001) and improving neurological deficit scores by 37.9% (95% CI 29.0–46.8%, p < 0.0001). This finding is in line with the findings of other potential agents against stroke, which decrease infarct volume and improve functional outcome by 24% on average in experimental studies (Sena et al. 2007; McCann et al. 2016; Schmidt-Pogoda et al. 2020). Due to the high risk of bias and low quality of evidence, which are common problems in preclinical studies, the efficacy of stroke treatments has declined from experimental to clinical trials (Macleod et al. 2004; Sena et al. 2007; McCann et al. 2016; Schmidt-Pogoda et al. 2020). Given the high risk of bias of the studies included in our meta-analyses, H2S is also likely to be less effective in clinical studies than in preclinical studies. Furthermore, functional outcome, the primary endpoint for clinical trials, has been evaluated in preclinical H2S stroke studies by using different methods, a situation that further jeopardizes the translation of animal studies. Twenty studies evaluating neurological deficits utilized various functional scales, including the Longa, Garcia, mNSS, Bederson, and Philips scoring systems, each covering different aspects of neurological function. Since Longa was the most commonly used scale, we performed pooled analyses including only studies using the Longa scale to increase the validity of the meta-analysis. Although this approach reduces between-study heterogeneity, it limits the power of the analysis due to the inclusion of only nine studies.
Another piece of evidence for the neuroprotective effect of H2S was a reduction in the brain water content, which was reported in nine studies using the wet-dry weight method. Brain water content is a measure commonly used in experimental animal stroke studies to assess cerebral edema, which occurs as a result of ischemia-induced inflammation and breakdown of the blood-brain barrier (Kozler et al. 2022). In the context of stroke research, cerebral edema is a significant concern because it can contribute to secondary damage and worsen outcomes following a stroke (Chen et al. 2021). H2S caused a small but significant reduction in brain water content (3.2%, 95% CI 1.4–4.9%, p = 0.0014) when the findings of the nine studies were pooled. As noted by Keep et al. (2012), when the brain water content is calculated in % using the wet-dry weight method, a “small” change in the % water content reflects a large change in the swelling of the tissue. Therefore, keeping in mind the high between-study heterogeneity (I2 = 94.6%, p < 0.0001), H2S significantly reduced brain edema after stroke.
Mechanisms of the neuroprotective effects of H2S
Research in recent decades has suggested that exogenous H2S and H2S donors exert neuroprotective effects on animal stroke models by inhibiting apoptosis, inflammation, oxidative stress, mitochondrial dysfunction, blood-brain barrier leakage, and disrupted cerebrovascular homeostasis, all of which contribute to ischemia and reperfusion injury in the brain (Dou et al. 2016; Chan and Wong 2017; Gopalakrishnan et al. 2019; Narne et al. 2019; Ding et al. 2023) (Fig. 7). The studies included in the meta-analysis evaluated these pathways by measuring various biomarkers. Among these, the ones for which the effect sizes could be pooled were the apoptosis markers TUNEL and caspase-3 and the inflammation markers IL-1beta and TNF-alpha. TUNEL was the only biomarker for which H2S significantly reduced the pooled results; caspase-3, IL-1beta, and TNF-alpha were not significantly affected. The fact that H2S does not affect proinflammatory cytokines but reduces TUNEL staining suggests that its antiapoptotic effect is more important for its neuroprotective effect than its anti-inflammatory effect. The observation that H2S decreases the number of TUNEL-positive cells without altering caspase-3 expression has multiple probable interpretations (Didenko et al. 2002). The antiapoptotic effect of H2S may involve caspase-independent apoptotic pathways or an early/intermediate stage of apoptosis. Since TUNEL staining could also be associated with other forms of cell death, such as necrosis or autophagy, the effect of H2S on TUNEL may also be explained by its inhibitory effect on autophagy induced by stroke (Chen et al. 2014).
Source of heterogeneity
To identify the sources of high heterogeneity between studies, we performed subgroup analyses to assess the effect of parameters related to the stroke model (e.g., duration of ischemia), treatment (e.g., type, dose, and time of H2S administration), and time of outcome assessment on the overall treatment effect. None of these parameters had a significant impact on heterogeneity. However, although not at a statistically significant level, H2S donors other than NaHS tended to be more effective in the stroke model. Because many different H2S donors were used in the studies, we were unable to obtain a sufficient number of studies for each donor to draw conclusions on the best donor effective against stroke. However, we observed that dual nitric oxide and H2S donors, such as 8e and 8d (Yin et al. 2016), and slow H2S-releasing agents, such as anethole dithiolethione (ADT) (Powell et al. 2018), are more effective than NaHS in preclinical stroke models. In further animal studies on stroke, we suggest using these or similar H2S donors.
Study quality and risk of bias
Compared with the meta-analyses of preclinical stroke studies in the literature, the quality of the studies included in our model was low (García-Bonilla et al. 2012; Schmidt et al. 2014; McCann et al. 2016; Boboc et al. 2023; Li et al. 2023). In previous studies, the median quality scores ranged between 4 and 7 out of 10 and between 6 and 11 out of 15, whereas the median score for the 34 studies in our analysis was 5 out of 15. Considering that most of the studies were published in 2015 and later, it is surprising that despite all efforts, preclinical stroke studies are still far from the expected quality. Low experimental and reporting quality creates a high risk of bias, making the reliability of the findings questionable. The most critical components for bias in stroke experiments (Sena et al. 2007), which are randomization of group allocation and blinded assessment of outcome, were reported in only 65% and 50% of the studies, respectively. Additionally, none of the studies reported any concern about avoiding the intrinsic neuroprotective effects of anesthesia. Five studies did not specify the anesthetics used. Chloral hydrate (n = 14), isoflurane (n = 12), halothane (n = 1), ketamine (n = 1), and sodium pentobarbital (n = 1) were also used. Although the CAMARADES checklist emphasizes that ketamine is the only anesthetic to avoid its neuroprotective effect (Sena et al. 2007), as there is evidence in the literature for neuroprotective effects of other anesthetics as well (Carbone and Austin 2016), we considered all studies to have a high risk of bias for this item. The lack of sample size calculations is another factor that increases the risk of bias. None of the studies reported any calculation or explanation for the sample size, which can lead to several drawbacks and limitations in the study design and interpretation of results, such as insufficient statistical power, inaccurate effect size estimates, increased type 1 and 2 errors, limited generalizability of the study findings, and increased ethical considerations (Percie du Sert et al. 2020). The use of male animals only, no use of animals with comorbidities, and no cotreatment were other factors that reduced the clinical and translational validity of the studies included in the meta-analysis.
In the subgroup analysis in which we evaluated the effect of the study quality score and risk of bias parameters on heterogeneity, surprisingly, studies reporting randomization and blinding and those with high-quality scores had larger effect sizes, accounting for 13.3%, 1.8%, and 3.6% of the heterogeneity, respectively. Although the p value for only randomization is significant according to the conventional significance threshold, others still indicate a difference at the trend level. The observation that randomization and blinding increase the effect size in an experimental animal study may seem counterintuitive and raise questions about the validity of the included studies. Considering that randomization and blinding correct the impact of sampling variability, selection bias, systemic errors, and confounding variables, we may also suggest that the effect of H2S on stroke is even greater than the estimates (McGough and Faraone 2009). However, given the poor study quality and small sample size, it is more plausible to suggest that randomization might result in seemingly large effects due to the limited number of subjects and insufficient study quality.
Publication bias
Another unexpected finding of the present meta-analysis was the symmetric funnel plot and nonsignificant Egger’s regression test, while the studies included in the model had a high risk of bias. This finding may suggest that publication bias may not be a major concern in our meta-analysis. However, it is crucial to note that funnel plot asymmetry is not solely indicative of publication bias; other factors can also contribute to asymmetry (Page et al. 2021). In our case, funnel plot symmetry may reflect genuine heterogeneity in the effect sizes across studies. A high risk of bias in individual studies may have contributed to this heterogeneity. In the absence of publication bias, heterogeneity in effect sizes may arise from variations in study design, animal models, interventions, or other biological factors. It is also possible that if a high risk of bias within individual studies is evenly distributed across studies and not selectively reported, it may not lead to funnel plot asymmetry.
Limitations
The main limitation of this meta-analysis is the high between-study heterogeneity, which decreases the power and reliability of the pooled effect sizes, making it challenging to detect a true effect of H2S in stroke patients. Subgroup analysis and risk of bias assessment revealed that the possible sources of heterogeneity were the various H2S donors used in the studies and the low quality of evidence. Although the pooled neuroprotective effect of H2S against stroke seems large, the true effect size needs to be confirmed in further well-designed experimental studies.
Conclusions and recommendations for further studies
H2S significantly reduces the stroke-induced infarct area, brain water content, and neurological deficit, primarily through early antiapoptotic effects. These promising findings are limited by the low quality of evidence and the high risk of bias represented by high heterogeneity in the effect sizes across studies. Despite all the efforts to standardize preclinical stroke studies and to increase translational success (Sena et al. 2007; Hooijmans et al. 2014; Schmidt-Pogoda et al. 2020), it is noteworthy that the internal and external validity of the animal studies evaluating the effect of H2S in stroke is low. When the studies included in our analysis are considered in the light of the CAMARADES, STAIR, and IMPROVE guidelines (Sena et al. 2007; Fisher et al. 2009; Percie du Sert et al. 2017), our specific recommendations for the future preclinical stroke studies on H2S are as follows:
-
Use a valid ischemic stroke model
-
Increase translation of findings by including both sexes, different age groups, and animals with comorbidities
-
Perform an accurate sample size calculation before the experiments
-
Report animal loss with reasons
-
Randomly allocate animals to the experimental groups
-
Blindly assess the outcome
-
Evaluate functional outcome, a more clinically relevant endpoint than stroke volume
-
Evaluate long-term effect of H2S
-
Compare H2S with current or emerging treatment options for ischemic stroke
-
Report transparently
In conclusion, although H2S seems promising against stroke, the available preclinical evidence is far from providing a basis for further clinical studies. Preclinical studies that provide high-quality evidence on the effect of H2S in stroke patients are still needed.
Data availability
All the data are available within each publication and in supplementary files.
References
Bahor Z, Liao J, Currie G, Ayder C, Macleod M, McCann SK, Bannach-Brown A, Wever K, Soliman N, Wang Q, Doran-Constant L, Young L, Sena ES, Sena C (2021) Development and uptake of an online systematic review platform: the early years of the CAMARADES Systematic Review Facility (SyRF). BMJ Open Sci 5(1):e100103. https://doi.org/10.1136/bmjos-2020-100103
Bath PM, Gray LJ, Bath AJ, Buchan A, Miyata T, Green AR (2009) Effects of NXY-059 in experimental stroke: an individual animal meta-analysis. Br J Pharmacol 157(7):1157–1171. https://doi.org/10.1111/j.1476-5381.2009.00196.x
Boboc IKS, Rotaru-Zavaleanu AD, Calina D, Albu CV, Catalin B, Turcu-Stiolica A (2023) A preclinical systematic review and meta-analysis of behavior testing in mice models of ischemic stroke. Life (Basel) 13(2):567. https://doi.org/10.3390/life13020567
Carbone L, Austin J (2016) Pain and laboratory animals: publication practices for better data reproducibility and better animal welfare. PLoS One 11(5):e0155001. https://doi.org/10.1371/journal.pone.0155001
Chan SJ, Wong PT (2017) Hydrogen sulfide in stroke: protective or deleterious? Neurochem Int 105:1–10. https://doi.org/10.1016/j.neuint.2016.11.015
Chen S, Shao L, Ma L (2021) Cerebral edema formation after stroke: emphasis on blood-brain barrier and the lymphatic drainage system of the brain. Front Cell Neurosci 15:716825. https://doi.org/10.3389/fncel.2021.716825
Chen W, Sun Y, Liu K, Sun X (2014) Autophagy: a double-edged sword for neuronal survival after cerebral ischemia. Neural Regen Res 9(12):1210–1216. https://doi.org/10.4103/1673-5374.135329
Clark JM, Sanders S, Carter M, Honeyman D, Cleo G, Auld Y, Booth D, Condron P, Dalais C, Bateup S, Linthwaite B, May N, Munn J, Ramsay L, Rickett K, Rutter C, Smith A, Sondergeld P, Wallin M, Jones M, Beller E (2020) Improving the translation of search strategies using the Polyglot Search Translator: a randomized controlled trial. J Med Libr Assoc 108(2):195–207. https://doi.org/10.5195/jmla.2020.834
de Vries RB, Hooijmans CR, Tillema A, Leenaars M, Ritskes-Hoitinga M (2014) Updated version of the Embase search filter for animal studies. Lab Anim 48(1):88. https://doi.org/10.1177/0023677213494374
Didenko VV, Ngo H, Minchew CL, Boudreaux DJ, Widmayer MA, Baskin DS (2002) Caspase-3-dependent and -independent apoptosis in focal brain ischemia. Mol Med 8(7):347–352
Ding JS, Zhang Y, Wang TY, Li X, Ma C, Xu ZM, Sun Q, Xu X, Chen G (2023) Therapeutic applications of hydrogen sulfide and novel donors for cerebral ischemic stroke: a narrative review. Med Gas Res 13(1):7–9. https://doi.org/10.4103/2045-9912.350863
Dirnagl U, Macleod MR (2009) Stroke research at a road block: the streets from adversity should be paved with meta-analysis and good laboratory practice. Br J Pharmacol 157(7):1154–1156. https://doi.org/10.1111/j.1476-5381.2009.00211.x
Dou Y, Wang Z, Chen G (2016) The role of hydrogen sulfide in stroke. Med Gas Res 6(2):79–84. https://doi.org/10.4103/2045-9912.184717
Emre Aydıngöz S, Teimoori A, Orhan HG, Efe OE, Kibaroğlu S, Erdem ŞR (2023) Effect of hydrogen sulfide on ischemia-reperfusion injury of kidney: a systematic review and meta-analysis of in vivo animal studies. Eur J Pharmacol 943:175564. https://doi.org/10.1016/j.ejphar.2023.175564
Fan J, Du J, Zhang Z, Shi W, Hu B, Hu J, Xue Y, Li H, Ji W, Zhuang J, Lv P, Cheng K, Chen K (2022) The protective effects of hydrogen sulfide new donor methyl S-(4-Fluorobenzyl)-N-(3,4,5-Trimethoxybenzoyl)-l-Cysteinate on the ischemic stroke. Molecules 27:1554
Fisher M, Feuerstein G, Howells DW, Hurn PD, Kent TA, Savitz SI, Lo EH (2009) Update of the stroke therapy academic industry roundtable preclinical recommendations. Stroke 40(6):2244–2250. https://doi.org/10.1161/strokeaha.108.541128
Florian B, Vintilescu R, Balseanu AT, Buga AM, Grisk O, Walker LC, Kessler C, Popa-Wagner A (2008) Long-term hypothermia reduces infarct volume in aged rats after focal ischemia. Neurosci Lett 438:180–185
García-Bonilla L, Campos M, Giralt D, Salat D, Chacón P, Hernández-Guillamon M, Rosell A, Montaner J (2012) Evidence for the efficacy of statins in animal stroke models: a meta-analysis. J Neurochem 122(2):233–243. https://doi.org/10.1111/j.1471-4159.2012.07773.x
Genc C, Tahta A, Erdag E, Dolas I, Sahin S, Karaoz E, Aras Y, Sabanci PA (2023) Human-derived hair follicle stem cells and hydrogen sulfide on focal cerebral ischemia model: a comparative evaluation of radiologic, neurobehavioral and immunohistochemical results. Brain Res 1799:148170. https://doi.org/10.1016/j.brainres.2022.148170
Gopalakrishnan P, Shrestha B, Kaskas AM, Green J, Alexander JS, Pattillo CB (2019) Hydrogen sulfide: therapeutic or injurious in ischemic stroke? Pathophysiology 26(1):1–10. https://doi.org/10.1016/j.pathophys.2018.10.005
Gheibi S, Aboutaleb N, Khaksari M, Kalalian-Moghaddam H, Vakili A, Asadi Y, Mehrjerdi FZ, Gheibi A (2014) Hydrogen sulfide protects the brain against ischemic reperfusion injury in a transient model of focal cerebral ischemia. J Mol Neurosci 54:264–270
Han X, Mao Z, Wang S, Xin Y, Li P, Maharjan S, Zhang B (2020) GYY4137 protects against MCAO via p38 MAPK mediated anti-apoptotic signaling pathways in rats. Brain Res Bull 158:59–65
Harrer MCP, Furukawa TA, Ebert DD (2021) Doing meta-analysis with R: a hands-on guide. Chapman & Hall/CRC Press, Boca Raton FL and London
Hooijmans CR, Rovers MM, de Vries RB, Leenaars M, Ritskes-Hoitinga M, Langendam MW (2014) SYRCLE’s risk of bias tool for animal studies. BMC Med Res Methodol 14:43. https://doi.org/10.1186/1471-2288-14-43
Jang H, Oh MY, Kim YJ, Choi IY, Yang HS, Ryu WS, Lee SH, Yoon BW (2014) Hydrogen sulfide treatment induces angiogenesis after cerebral ischemia. J Neurosci Res 92:1520–1528
Ji K, Xue L, Cheng J, Bai Y (2016) Preconditioning of H2S inhalation protects against cerebral ischemia/reperfusion injury by induction of HSP70 through PI3K/Akt/Nrf2 pathway. Brain Res Bull 121:68–74
Jia J, Jiao W, Wang G, Wu J, Huang Z, Zhang Y (2024) Drugs/agents for the treatment of ischemic stroke: advances and perspectives. Med Res Rev 44(3):975–1012. https://doi.org/10.1002/med.22009
Jia J, Li J, Cheng J (2019) H(2)S-based therapies for ischaemic stroke: opportunities and challenges. Stroke Vasc Neurol 4(2):63–66. https://doi.org/10.1136/svn-2018-000194
Jiang WW, Huang BS, Han Y, Deng LH, Wu LX (2017) Sodium hydrosulfide attenuates cerebral ischemia/reperfusion injury by suppressing overactivated autophagy in rats. FEBS Open Bio 7:1686–1695
Joseph C, Buga AM, Vintilescu R, Balseanu AT, Moldovan M, Junker H, Walker L, Lotze M, Popa-Wagner A (2012) Prolonged gaseous hypothermia prevents the upregulation of phagocytosis-specific protein annexin 1 and causes low-amplitude EEG activity in the aged rat brain after cerebral ischemia. J Cereb Blood Flow Metab 32:1632–1642
Keep RF, Hua Y, Xi G (2012) Brain water content. A misunderstood measurement? Transl Stroke Res 3(2):263–265. https://doi.org/10.1007/s12975-012-0152-2
Kimura H (2021) Hydrogen sulfide (H(2)S) and polysulfide (H(2)S(n)) signaling: the first 25 years. Biomolecules 11(6):896. https://doi.org/10.3390/biom11060896
Knapp G, Hartung J (2003) Improved tests for a random effects meta-regression with a single covariate. Stat Med 22(17):2693–2710. https://doi.org/10.1002/sim.1482
Kozler P, Marešová D, Pokorný J (2022) Determination of brain water content by dry/wet weight measurement for the detection of experimental brain edema. Physiol Res 71(S2):S277–s283. https://doi.org/10.33549/physiolres.934996
Li GF, Luo HK, Li LF, Zhang QZ, Xie LJ, Jiang H, Li LP, Hao N, Wang WW, Zhang JX (2012) Dual effects of hydrogen sulphide on focal cerebral ischaemic injury via modulation of oxidative stress-induced apoptosis. Clin Exp Pharmacol Physiol 39:765–771
Li XJ, Li CK, Wei LY, Lu N, Wang GH, Zhao HG, Li DL (2015) Hydrogen sulfide intervention in focal cerebral ischemia/reperfusion injury in rats. Neural Regen Res 10:932–937
Li X, Zhang J, Zhu X, Li X, Wang X, Li D (2016) Hydrogen sulfide protects focal cerebral ischemia-reperfusion injury in rats through the PI3K/Akt signaling pathway. Int J Clin Exp Pathol 9:5930–5936
Li P, Yin R, Chen Y, Chang J, Yang L, Liu X, Xu H, Zhang X, Wang S, Han Q, Wei J (2023) Engineered extracellular vesicles for ischemic stroke: a systematic review and meta-analysis of preclinical studies. J Nanobiotechnology 21(1):396. https://doi.org/10.1186/s12951-023-02114-8
Lin X, Yu S, Chen Y, Wu J, Zhao J, Zhao Y (2012) Neuroprotective effects of diallyl sulfide against transient focal cerebral ischemia via anti-apoptosis in rats. Neurol Res 34:32–37
Lin JJ, Chang T, Cai WK, Zhang Z, Yang YX, Sun C, Li ZY, Li WX (2015) Post-injury administration of allicin attenuates ischemic brain injury through sphingosine kinase 2: In vivo and in vitro studies. Neurochem Int 89:92–100
Liu H, Wang Y, Xiao Y, Hua Z, Cheng J, Jia J (2016) Hydrogen sulfide attenuates tissue plasminogen activator-induced cerebral hemorrhage following experimental stroke. Transl Stroke Res 7(3):209–219. https://doi.org/10.1007/s12975-016-0459-5
Longa EZ, Weinstein PR, Carlson S, Cummins R (1989) Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 20:84–91
Lyden PD (2021) Cerebroprotection for acute ischemic stroke: looking ahead. Stroke 52(9):3033–3044. https://doi.org/10.1161/strokeaha.121.032241
Macleod MR, O'Collins T, Howells DW, Donnan GA (2004) Pooling of animal experimental data reveals influence of study design and publication bias. Stroke 35(5):1203–1208. https://doi.org/10.1161/01.Str.0000125719.25853.20
Macleod MR, van der Worp HB, Sena ES, Howells DW, Dirnagl U, Donnan GA (2008) Evidence for the efficacy of NXY-059 in experimental focal cerebral ischaemia is confounded by study quality. Stroke 39(10):2824–2829. https://doi.org/10.1161/strokeaha.108.515957
McCann SK, Cramond F, Macleod MR, Sena ES (2016) Systematic review and meta-analysis of the efficacy of interleukin-1 receptor antagonist in animal models of stroke: an update. Transl Stroke Res 7(5):395–406. https://doi.org/10.1007/s12975-016-0489-z
McGough JJ, Faraone SV (2009) Estimating the size of treatment effects: moving beyond p values. Psychiatry (Edgmont) 6(10):21–29
McGuinness LA, Higgins JPT (2021) Risk-of-bias VISualization (robvis): an R package and Shiny web app for visualizing risk-of-bias assessments. Res Synth Methods 12(1):55–61. https://doi.org/10.1002/jrsm.1411
Mendonça BP, Cardoso JDS, Michels M, Vieira AC, Wendhausen D, Manfredini A, Singer M, Dal-Pizzol F, Dyson A (2020) Neuroprotective effects of ammonium tetrathiomolybdate, a slow-release sulfide donor, in a rodent model of regional stroke. Intensive Care Med Exp 8:13
Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 151(4):264–269, w264. https://doi.org/10.7326/0003-4819-151-4-200908180-00135
Narne P, Pandey V, Phanithi PB (2019) Role of nitric oxide and hydrogen sulfide in ischemic stroke and the emergent epigenetic underpinnings. Mol Neurobiol 56(3):1749–1769. https://doi.org/10.1007/s12035-018-1141-6
Numagami Y, Sato S, Ohnishi ST (1996) Attenuation of rat ischemic brain damage by aged garlic extracts: a possible protecting mechanism as antioxidants. Neurochem Int 29:135–143
Page MJ, Sterne JAC, Higgins JPT, Egger M (2021) Investigating and dealing with publication bias and other reporting biases in meta-analyses of health research: a review. Res Synth Methods 12(2):248–259. https://doi.org/10.1002/jrsm.1468
Percie du Sert N, Alfieri A, Allan SM, Carswell HV, Deuchar GA, Farr TD, Flecknell P, Gallagher L, Gibson CL, Haley MJ, Macleod MR, McColl BW, McCabe C, Morancho A, Moon LD, O'Neill MJ, Pérez de Puig I, Planas A, Ragan CI et al (2017) The IMPROVE guidelines (ischaemia models: procedural refinements of in vivo experiments). J Cereb Blood Flow Metab 37(11):3488–3517. https://doi.org/10.1177/0271678x17709185
Percie du Sert N, Hurst V, Ahluwalia A, Alam S, Avey MT, Baker M, Browne WJ, Clark A, Cuthill IC, Dirnagl U, Emerson M, Garner P, Holgate ST, Howells DW, Karp NA, Lazic SE, Lidster K, MacCallum CJ, Macleod M et al (2020) The ARRIVE guidelines 2.0: updated guidelines for reporting animal research. PLoS Biol 18(7):e3000410. https://doi.org/10.1371/journal.pbio.3000410
Pomierny B, Krzyżanowska W, Jurczyk J, Skórkowska A, Strach B, Szafarz M, Przejczowska-Pomierny K, Torregrossa R, Whiteman M, Marcinkowska M, Pera J, Budziszewska B (2021) The slow-releasing and mitochondria-targeted hydrogen sulfide (H(2)S) delivery molecule ap39 induces brain tolerance to ischemia. Int J Mol Sci 22:7816
Powell CR, Dillon KM, Matson JB (2018) A review of hydrogen sulfide (H(2)S) donors: chemistry and potential therapeutic applications. Biochem Pharmacol 149:110–123. https://doi.org/10.1016/j.bcp.2017.11.014
Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, Biller J, Brown M, Demaerschalk BM, Hoh B, Jauch EC, Kidwell CS, Leslie-Mazwi TM, Ovbiagele B, Scott PA, Sheth KN, Southerland AM, Summers DV, Tirschwell DL (2018) 2018 Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 49(3):e46–e110. https://doi.org/10.1161/str.0000000000000158
Qu K, Chen CP, Halliwell B, Moore PK, Wong PT (2006) Hydrogen sulfide is a mediator of cerebral ischemic damage. Stroke 37:889–893
Saini V, Guada L, Yavagal DR (2021) Global epidemiology of stroke and access to acute ischemic stroke interventions. Neurology 97(20 Suppl 2):S6–s16. https://doi.org/10.1212/wnl.0000000000012781
Schmidt A, Wellmann J, Schilling M, Strecker JK, Sommer C, Schäbitz WR, Diederich K, Minnerup J (2014) Meta-analysis of the efficacy of different training strategies in animal models of ischemic stroke. Stroke 45(1):239–247. https://doi.org/10.1161/strokeaha.113.002048
Schmidt-Pogoda A, Bonberg N, Koecke MHM, Strecker JK, Wellmann J, Bruckmann NM, Beuker C, Schäbitz WR, Meuth SG, Wiendl H, Minnerup H, Minnerup J (2020) Why most acute stroke studies are positive in animals but not in patients: a systematic comparison of preclinical, early phase, and phase 3 clinical trials of neuroprotective agents. Ann Neurol 87(1):40–51. https://doi.org/10.1002/ana.25643
Sena E, van der Worp HB, Howells D, Macleod M (2007) How can we improve the pre-clinical development of drugs for stroke? Trends Neurosci 30(9):433–439. https://doi.org/10.1016/j.tins.2007.06.009
Sena ES, van der Worp HB, Bath PM, Howells DW, Macleod MR (2010) Publication bias in reports of animal stroke studies leads to major overstatement of efficacy. PLoS Biol 8(3):e1000344. https://doi.org/10.1371/journal.pbio.1000344
Shi HQ, Zhang Y, Cheng MH, Fan BS, Tian JS, Yu JG, Chen B (2016) Sodium sulfide, a hydrogen sulfide-releasing molecule, attenuates acute cerebral ischemia in rats. CNS Neurosci Ther 22:625–632
Shui M, Liu X, Zhu Y, Wang Y (2016) Exogenous hydrogen sulfide attenuates cerebral ischemia-reperfusion injury by inhibiting autophagy in mice. Can J Physiol Pharmacol 94:1187–1192
Soliman N, Rice ASC, Vollert J (2020) A practical guide to preclinical systematic review and meta-analysis. Pain 161(9):1949–1954. https://doi.org/10.1097/j.pain.0000000000001974
Sun Y, Zhang Y, Li Y, Cheng J, Chen S, Xiao Y, Ao G (2016) Synthesis and biological evaluation of novel hydrogen sulfide releasing nicotinic acid derivatives. Bioorg Med Chem 24:5368–5373
Tao L, Yu Q, Zhao P, Yang Q, Wang B, Yang Y, Kuai J, Ding Q (2019) Preconditioning with hydrogen sulfide ameliorates cerebral ischemia/reperfusion injury in a mouse model of transient middle cerebral artery occlusion. Chem Biol Interact 310:108738
van der Mierden S, Hooijmans CR, Tillema AH, Rehn S, Bleich A, Leenaars CH (2022) Laboratory animals search filter for different literature databases: PubMed, Embase, Web of Science and PsycINFO. Lab Anim 56(3):279–286. https://doi.org/10.1177/00236772211045485
Vesterinen HM, Sena ES, Egan KJ, Hirst TC, Churolov L, Currie GL, Antonic A, Howells DW, Macleod MR (2014) Meta-analysis of data from animal studies: a practical guide. J Neurosci Methods 221:92–102. https://doi.org/10.1016/j.jneumeth.2013.09.010
Viechtbauer W (2010) Conducting meta-analyses in R with the metafor package. J Stat Software 36:1–48
Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Delling FN, Djousse L, Elkind MSV, Ferguson JF, Fornage M, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT et al (2020) Heart disease and stroke statistics-2020 update: a report from the American heart Association. Circulation 141(9):e139–e596. https://doi.org/10.1161/cir.0000000000000757
Wang Y, Jia J, Ao G, Hu L, Liu H, Xiao Y, Du H, Alkayed NJ, Liu CF, Cheng J (2014) Hydrogen sulfide protects bloodbrain barrier integrity following cerebral ischemia. J Neurochem 129:827–838
Wang L, Wang X, Li T, Zhang Y, Ji H (2018) 8e protects against acute cerebral ischemia by inhibition of PI3Kγ-mediated superoxide generation in microglia. Molecules 23:2828
Wang X, Piantadosi S, Le-Rademacher J, Mandrekar SJ (2021) Statistical considerations for subgroup analyses. J Thorac Oncol 16(3):375–380. https://doi.org/10.1016/j.jtho.2020.12.008
Wen JY, Wang M, Li YN, Jiang HH, Sun XJ, Chen ZW (2018) Vascular protection of hydrogen sulfide on cerebral ischemia/reperfusion injury in rats. Front Neurol 9:779
Wei X, Zhang B, Cheng L, Chi M, Deng L, Pan H, Yao X, Wang G (2015) Hydrogen sulfide induces neuroprotection against experimental stroke in rats by down-regulation of AQP4 via activating PKC. Brain Res 1622: 292-299
Woo CW, Kwon JI, Kim KW, Kim JK, Jeon SB, Jung SC, Choi CG, Kim ST, Kim J, Ham SJ, Shim WH, Sung YS, Ha HK, Choi Y, Woo DC (2017) The administration of hydrogen sulphide prior to ischemic reperfusion has neuroprotective effects in an acute stroke model. PLoS One 12:e0187910
Yang KL, Li WH, Liu YJ, Wei YJ, Ren YK, Mai CD, Zhang SY, Zuo Y, Sun ZZ, Li DL, Yang CH (2022) Hydrogen sulfide attenuates neuroinflammation by inhibiting the NLRP3/caspase-1/GSDMD pathway in retina or brain neuron following rat ischemia/reperfusion. Brain Sci 12:1245
Yin J, Zeng QH, Shen Q, Yang XS (2013) Neuroprotective mechanism of hydrogen sulfide after cerebral ischemiareperfusion in rats. Zhonghua Yi Xue Za Zhi 93:868–872
Yin W, Lan L, Huang Z, Ji J, Fang J, Wang X, Ji H, Peng S, Xu J, Zhang Y (2016) Discovery of a ring-opened derivative of 3-n-butylphthalide bearing NO/H2S-donating moieties as a potential anti-ischemic stroke agent. Eur J Med Chem 115:369–380. https://doi.org/10.1016/j.ejmech.2016.03.044
Yu Q, Lu Z, Tao L, Yang L, Guo Y, Yang Y, Sun X, Ding Q (2015) ROS-dependent neuroprotective effects of NaHS in ischemia brain injury involves the PARP/AIF pathway. Cell Physiol Biochem 36:1539–1551
Zhang B, Li F, Zhao W, Li J, Li Q, Wang W (2015) Protective effects of allicin against ischemic stroke in a rat model of middle cerebral artery occlusion. Mol Med Rep 12:3734–3738
Zhang M, Wu X, Xu Y, He M, Yang J, Li J, Li Y, Ao G, Cheng J, Jia J (2017) The cystathionine β-synthase/hydrogen sulfide pathway contributes to microglia-mediated neuroinflammation following cerebral ischemia. Brain Behav Immun 66:332–346
Zhu Y, Shui M, Liu X, Hu W, Wang Y (2017) Increased autophagic degradation contributes to the neuroprotection of hydrogen sulfide against cerebral ischemia/reperfusion injury. Metab Brain Dis 32:1449–1458
Zwetsloot PP, Van Der Naald M, Sena ES, Howells DW, IntHout J, De Groot JA, Chamuleau SA, MacLeod MR, Wever KE (2017) Standardized mean differences cause funnel plot distortion in publication bias assessments. Elife 6:e24260. https://doi.org/10.7554/eLife.24260
Acknowledgements
We would like to thank Sofija Vojvodic and Daniel Schulze from CAMARADES Berlin, QUEST Center, Berlin Institute of Health at Charité Universitätsmedizin, for their help while searching the EMBASE database and conducting the statistical analysis.
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK). This study was supported by the Baskent University Research Fund (project no: DA23/02).
Author information
Authors and Affiliations
Contributions
SEA: conceptualization, study design, literature search, data collection, discrepancy resolving, analysis, manuscript writing. AT: literature search, data collection, analysis, manuscript writing. HGO: literature search, data collection, analysis, manuscript writing. NZ: data collection. ED: data collection. All authors had read and approved the final version of the manuscript. The authors confirm that no paper mill and artificial intelligence was used.
Corresponding author
Ethics declarations
Ethics approval
The study was not subject to ethics committee approval because it did not involve any experimental or clinical intervention.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
Supplement 1. The PRISMA checklist (DOCX 31 kb)
ESM 2
Supplement 2. Search criteria (DOCX 42 kb)
ESM 3
Supplement 3. Extracted data (XLSX 153 kb)
ESM 4
Supplement 4. R codes (PDF 143 kb)
ESM 5
Supplement 5. Summary of studies (DOCX 3.75 mb)
ESM 6
Supplement 6. Leave-one-out analysis results (DOCX 16 kb)
ESM 7
Supplement 7. Forest plot showing the effect of various H2S donors on the infarct area (JPG 1117 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Emre Aydıngöz, S., Teimoori, A., Orhan, H.G. et al. A meta-analysis of animal studies evaluating the effect of hydrogen sulfide on ischemic stroke: is the preclinical evidence sufficient to move forward?. Naunyn-Schmiedeberg's Arch Pharmacol (2024). https://doi.org/10.1007/s00210-024-03291-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00210-024-03291-5