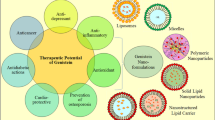
Abstract
Genistein, a commonly occurring isoflavone, has recently gained popularity owing to its ever-expanding spectrum of pharmacological benefits. In addition to health benefits such as improved bone health and reduced postmenopausal complications owing to its phytoestrogen properties, it has been widely evaluated for its anti-cancer potential. Several studies have established the potential for its usage in the management of breast, lung, and prostate cancers, and its usage has significantly evolved from early applications in traditional systems of medicine. This review offers an insight into its current status of usage, the chemistry, and pharmacokinetics of the molecule, an exploration of its apoptotic mechanisms in cancer management, and opportunities for synergism to improve therapeutic outcomes. In addition to this, the authors have presented an overview of recent clinical trials, to offer an understanding of contemporary studies and explore prospects for a greater number of focused trials, moving forward. Advancements in the application of nanotechnology as a strategy to improve safety and efficacy have also been highlighted, with a brief discussion of results from safety and toxicology studies.



Similar content being viewed by others
Data availability
This document includes citations for all the data that were analyzed throughout the literature review.
References
Abdulridha MK, Al-Marzoqi AH, Al-Awsi GRL, Mubarak SM, Heidarifard M, Ghasemian A (2020) Anticancer effects of herbal medicine compounds and novel formulations: a literature review. J Gastrointest Cancer 51:765–773
Ahmad A, Biersack B, Li Y, et al (2013) Deregulation of PI3K/Akt/mTOR signaling pathways by isoflavones and its implication in cancer treatment. Anti-cancer agents in medicinal chemistry (formerly current medicinal chemistry-anti-cancer agents) 13:1014–1024
Akimoto T, Nonaka T, Ishikawa H, et al (2001) Genistein, a tyrosine kinase inhibitor, enhanced radiosensitivity in human esophageal cancer cell lines in vitro: possible involvement of inhibition of survival signal transduction pathways. International Journal of Radiation Oncology* Biology* Physics 50:195–201
Alorda-Clara M, Torrens-Mas M, Morla-Barcelo PM et al (2022) High concentrations of genistein decrease cell viability depending on oxidative stress and inflammation in colon cancer cell lines. Int J Mol Sci 23:7526
Arora L, Mohan CD, Yang MH et al (2021) Tris (dibenzylideneacetone) dipalladium (0)(Tris DBA) abrogates tumor progression in hepatocellular carcinoma and multiple myeloma preclinical models by regulating the STAT3 signaling pathway. Cancers 13:5479
Baburajeev C, Mohan CD, Patil GS et al (2016) Nano-cuprous oxide catalyzed one-pot synthesis of a carbazole-based STAT3 inhibitor: a facile approach via intramolecular C-N bond formation reactions. RSC Adv 6:36775–36785
Bi Y-l, Min M, Shen W, Liu Y (2018) Genistein induced anticancer effects on pancreatic cancer cell lines involves mitochondrial apoptosis, G0/G1cell cycle arrest and regulation of STAT3 signalling pathway. Phytomedicine 39:10–16
Bosland MC, Enk E, Schmoll J et al (2021) Soy protein supplementation in men following radical prostatectomy: a 2-year randomized, placebo-controlled clinical trial. Am J Clin Nutr 113:821–831
Bosland MC, Schmoll J, Watanabe H, Randolph C, Kato I (2022) Randomized, placebo-controlled six-month intervention study of soy protein isolate in men with biochemical recurrence after radical prostatectomy: a pilot study. Nutr Cancer 74:555–564
Budihardjo I, Oliver H, Lutter M, Luo X, Wang X (1999) Biochemical pathways of caspase activation during apoptosis. Annu Rev Cell Dev Biol 15:269–290
Chan L, Pang Y, Wang Y et al (2022) Genistein-induced mitochondrial dysfunction and FOXO3a/PUMA expression in non-small lung cancer cells. Pharm Biol 60:1876–1883
Chen M, Wang J (2002) Initiator caspases in apoptosis signaling pathways. Apoptosis 7:313–319
Chen J, Duan Y, Zhang X, Ye Y, Ge B, Chen J (2015) Genistein induces apoptosis by the inactivation of the IGF-1R/p-Akt signaling pathway in MCF-7 human breast cancer cells. Food Funct 6:995–1000
Chen L-R, Ko N-Y, Chen K-H (2019b) Isoflavone supplements for menopausal women: a systematic review. Nutrients 11:2649
Chen C, Wang Y, Chen S et al (2020) Genistein inhibits migration and invasion of cervical cancer HeLa cells by regulating FAK-paxillin and MAPK signaling pathways. Taiwan J Obstet Gynecol 59:403–408
Chen M, Samuel VP, Wu Y, et al (2019a) Nrf2/HO-1 mediated protective activity of genistein against doxorubicin-induced cardiac toxicity. Journal of Environmental Pathology, Toxicology and Oncology 38
Cheng W-X, Huang H, Chen J-H et al (2020) Genistein inhibits angiogenesis developed during rheumatoid arthritis through the IL-6/JAK2/STAT3/VEGF signalling pathway. Journal of Orthopaedic Translation 22:92–100
Chiawpanit C, Panwong S, Sawasdee N, Yenchitsomanus P-t, Panya A (2022) Genistein sensitizes human cholangiocarcinoma cell lines to be susceptible to natural killer cells. Biology 11:1098
Chinni SR, Alhasan SA, Multani AS, Pathak S, Sarkar FH (2003) Pleotropic effects of genistein on MCF-7 breast cancer cells. Int J Mol Med 12:29–34
Dai X, Zhang J, Arfuso F et al (2015) Targeting TNF-related apoptosis-inducing ligand (TRAIL) receptor by natural products as a potential therapeutic approach for cancer therapy. Exp Biol Med 240:760–773
Dev A, Sardoiwala MN, Kushwaha AC, Karmakar S, Choudhury SR (2021) Genistein nanoformulation promotes selective apoptosis in oral squamous cell carcinoma through repression of 3PK-EZH2 signalling pathway. Phytomedicine 80:153386
Dutta S, Moses JA, Anandharamakrishnan C (2018) Encapsulation of nutraceutical ingredients in liposomes and their potential for cancer treatment. Nutr Cancer 70:1184–1198
Elmowafy M, Shalaby K, Elkomy M et al (2022) Impact of highly phospholipid-containing lipid nanocarriers on oral bioavailability and pharmacodynamics performance of genistein. Pharm Dev Technol 27:435–447
Fan Y, Liu B, Chen F et al (2021) Hepcidin upregulation in lung cancer: a potential therapeutic target associated with immune infiltration. Front Immunol 12:612144
Ferrado JB, Perez AA, Baravalle ME, Renna MS, Ortega HH, Santiago LG (2021) Genistein loaded in self-assembled bovine serum albumin nanovehicles and their effects on mouse mammary adenocarcinoma cells. Colloids Surf, B 204:111777
Ferrado JB, Perez AA, Menegon M et al (2023) PEGylation of genistein-loaded bovine serum albumin nanoparticles and its effect on in vitro cell viability and genotoxicity properties. Colloids Surf, B 222:113082
Gao J, Xia R, Chen J et al (2020) Inhibition of esophageal-carcinoma cell proliferation by genistein via suppression of JAK1/2-STAT3 and AKT/MDM2/p53 signaling pathways. Aging (albany NY) 12:6240
Garbiec E, Cielecka-Piontek J, Kowalówka M, Hołubiec M, Zalewski P (2022) Genistein—opportunities related to an interesting molecule of natural origin. Molecules 27:815
Ghasemi Goorbandi R, Mohammadi MR, Malekzadeh K (2020) Synthesizing efficacious genistein in conjugation with superparamagnetic Fe3O4 decorated with bio-compatible carboxymethylated chitosan against acute leukemia lymphoma. Biomater. Res. 24:1–13
Girisa S, Shabnam B, Monisha J et al (2019) Potential of zerumbone as an anti-cancer agent. Molecules 24:734
Godschalk RW, Janssen MC, Vanhees K, van Doorn SBvW, van Schooten F-J (2022) Maternal exposure to genistein during pregnancy and oxidative DNA damage in testes of male mouse offspring. Frontiers in nutrition 9
Gong L, Li Y, Nedeljkovic-Kurepa A, Sarkar FH (2003) Inactivation of NF-κB by genistein is mediated via Akt signaling pathway in breast cancer cells. Oncogene 22:4702–4709
Hakami T, Mahmoud M, de Juan E, Cooney M (2021) Pharmacokinetics of genistein distribution in blood and retinas of diabetic and non-diabetic rats. Drug Metab Pharmacokinet 39:100404
Hazafa A, Rehman K-U-, Jahan N, Jabeen Z. (2020) The role of polyphenol (flavonoids) compounds in the treatment of cancer cells. Nutr Cancer 72:386–397
He H, Chen L, Zhai M, Chen JZ (2009) Genistein down-regulates the constitutive activation of nuclear factor-κB in human multiple myeloma cells, leading to suppression of proliferation and induction of apoptosis. Phytotherapy Research: an International Journal Devoted to Pharmacological and Toxicological Evaluation of Natural Product Derivatives 23:868–873
Hsiao YC, Peng SF, Lai KC et al (2019) Genistein induces apoptosis in vitro and has antitumor activity against human leukemia HL-60 cancer cell xenograft growth in vivo. Environ Toxicol 34:443–456
Hsiao YC, Chueh FS, Ma YS et al (2021) Genistein enhances the effects of L-asparaginase on inducing cell apoptosis in human leukemia cancer HL-60 cells. Environ Toxicol 36:764–772
Hsieh P-L, Liao Y-W, Hsieh C-W, Chen P-N, Yu C-C (2020) Soy isoflavone genistein impedes cancer stemness and mesenchymal transition in head and neck cancer through activating miR-34a/RTCB axis. Nutrients 12:1924
Hu C-D, Choo R, Huang J (2015) Neuroendocrine differentiation in prostate cancer: a mechanism of radioresistance and treatment failure. Front Oncol 5:90
Hu Q-p, Yan H-x, Peng F et al (2021) Genistein protects epilepsy-induced brain injury through regulating the JAK2/STAT3 and Keap1/Nrf2 signaling pathways in the developing rats. Eur J Pharmacol 912:174620
Islam A, Islam MS, Uddin MN, Hasan MMI, Akanda MR (2020) The potential health benefits of the isoflavone glycoside genistin. Arch Pharmacal Res 43:395–408
Jeong J-W, Lee HH, Han MH, Kim G-Y, Kim W-J, Choi YH (2014) Anti-inflammatory effects of genistein via suppression of the toll-like receptor 4-mediated signaling pathway in lipopolysaccharide-stimulated BV2 microglia. Chem Biol Interact 212:30–39
Ji Z, Huo C, Yang P (2020) Genistein inhibited the proliferation of kidney cancer cells via CDKN2a hypomethylation: role of abnormal apoptosis. Int Urol Nephrol 52:1049–1055
Jiang H, Fan J, Cheng L, Hu P, Liu R (2018) The anticancer activity of genistein is increased in estrogen receptor beta 1-positive breast cancer cells. OncoTargets Therapy: 8153–8163
Jin C-Y, Park C, Cheong J et al (2007) Genistein sensitizes TRAIL-resistant human gastric adenocarcinoma AGS cells through activation of caspase-3. Cancer Lett 257:56–64
Jin C-Y, Park C, Moon S-K et al (2009a) Genistein sensitizes human hepatocellular carcinoma cells to TRAIL-mediated apoptosis by enhancing Bid cleavage. Anticancer Drugs 20:713–722
Jin C-Y, Park C, Kim G-Y, Lee S-J, Kim W-J, Choi YH (2009b) Genistein enhances TRAIL-induced apoptosis through inhibition of p38 MAPK signaling in human hepatocellular carcinoma Hep3B cells. Chem Biol Interact 180:143–150
Jin C-Y, Park C, Park S-E, Hong S-H, Choi Y-H (2011) Enhancement of TRAIL-mediated apoptosis by genistein in human hepatocellular carcinoma Hep3B cells: roles of p38 MAPK signaling pathway. J. Life Sci. 21:1549–1557
Joshi H, Malik A, Aggarwal S, et al (2019) In-vitro detection of phytopathogenic fungal cell wall by polyclonal sera raised against trimethyl chitosan nanoparticles. International Journal of Nanomedicine: 10023–10033
Joshi H, Kumar G, Tuli HS, Mittal S (2023) Inhibition of cancer cell metastasis by nanotherapeutics: current achievements and future trends. Nanotherapeutics in Cancer. Jenny Stanford Publishing, pp. 161–209
Julián-Serrano S, Yuan F, Wheeler W et al (2021) Hepcidin-regulating iron metabolism genes and pancreatic ductal adenocarcinoma: a pathway analysis of genome-wide association studies. Am J Clin Nutr 114:1408–1417
Kamel NM, Helmy MW, Abdelfattah E-Z et al (2019) Inhalable dual-targeted hybrid lipid nanocore–protein shell composites for combined delivery of genistein and all-trans retinoic acid to lung cancer cells. ACS Biomater Sci Eng 6:71–87
Kaushik S, Shyam H, Agarwal S et al (2019) Genistein potentiates centchroman induced antineoplasticity in breast cancer via PI3K/Akt deactivation and ROS dependent induction of apoptosis. Life Sci 239:117073
Kim I-S (2021) Current perspectives on the beneficial effects of soybean isoflavones and their metabolites for humans. Antioxidants 10:1064
Kim I-S, Kim C-H, Yang W-S (2021) Physiologically active molecules and functional properties of soybeans in human health—a current perspective. Int J Mol Sci 22:4054
Křížová L, Dadáková K, Kašparovská J, Kašparovský T (2019) Isoflavones Molecules 24:1076
Landauer MR, Harvey AJ, Kaytor MD, Day RM (2019) Mechanism and therapeutic window of a genistein nanosuspension to protect against hematopoietic-acute radiation syndrome. J Radiat Res 60:308–317
Lavigne JA, Takahashi Y, Chandramouli GV et al (2008) Concentration-dependent effects of genistein on global gene expression in MCF-7 breast cancer cells: an oligo microarray study. Breast Cancer Res Treat 110:85–98
Lee SR, Kwon SW, Lee YH et al (2019a) Dietary intake of genistein suppresses hepatocellular carcinoma through AMPK-mediated apoptosis and anti-inflammation. BMC Cancer 19:1–12
Lee JH, Mohan CD, Basappa S et al (2019b) The IκB kinase inhibitor ACHP targets the STAT3 signaling pathway in human non-small cell lung carcinoma cells. Biomolecules 9:875
Lee JH, Rangappa S, Mohan CD et al (2019c) Brusatol, a Nrf2 inhibitor targets STAT3 signaling cascade in head and neck squamous cell carcinoma. Biomolecules 9:550
Lee JH, Mohan CD, Shanmugam MK et al (2020a) Vitexin abrogates invasion and survival of hepatocellular carcinoma cells through targeting STAT3 signaling pathway. Biochimie 175:58–68
Lee JH, Mohan CD, Deivasigamani A et al (2020b) Brusatol suppresses STAT3-driven metastasis by downregulating epithelial-mesenchymal transition in hepatocellular carcinoma. J Adv Res 26:83–94
Lee D, Kim J-Y, Kim H-W, Yoo J-E, Kang KS (2021) Combined beneficial effect of genistein and atorvastatin on adipogenesis in 3t3-L1 adipocytes. Biomolecules 11:1052
Li Y, Sarkar FH (2002) Inhibition of nuclear factor κB activation in PC3 cells by genistein is mediated via Akt signaling pathway. Clin Cancer Res 8:2369–2377
Li Y, Bhuiyan M, Sarkar FH (1999a) Induction of apoptosis and inhibition of c-erbB-2 in MDA-MB-435 cells by genistein. Int J Oncol 15:525–558
Li Y, Upadhyay S, Bhuiyan M, Sarkar FH (1999b) Induction of apoptosis in breast cancer cells MDA-MB-231 by genistein. Oncogene 18:3166–3172
Li Z, Li J, Mo B et al (2008) Genistein induces cell apoptosis in MDA-MB-231 breast cancer cells via the mitogen-activated protein kinase pathway. Toxicol in Vitro 22:1749–1753
Li K, Hong S, Lin S, Chen K (2020) Genistein inhibits the proliferation, migration and invasion of the squamous cell carcinoma cells via inhibition of MEK/ERK and JNK signalling pathways. J BU ON 25:1172–1177
Li J, Li J, Yue Y, et al (2014) Genistein suppresses tumor necrosis factor α-induced inflammation via modulating reactive oxygen species/Akt/nuclear factor κB and adenosine monophosphate-activated protein kinase signal pathways in human synoviocyte MH7A cells. Drug Design, Development Therapy:315–323
Lian F, Li Y, Bhuiyan M, Sarkar FH (1999) p53-independent apoptosis induced by genistein in lung cancer cells. Nutr Cancer 33:125–131
Lian JP, Word B, Taylor S, Hammons GJ, Lyn-Cook BD (2004) Modulation of the constitutive activated STAT3 transcription factor in pancreatic cancer prevention: effects of indole-3-carbinol (I3C) and genistein. Anticancer Res 24:133–138
Lian F, Bhuiyan M, Li YW, Wall N, Kraut M, Sarkar FH (1998) Genistein‐induced G2‐M arrest, p21WAF1 upregulation, and apoptosis in a non‐small‐cell lung cancer cell line
Liu X, Sun C, Jin X et al (2013) Genistein enhances the radiosensitivity of breast cancer cells via G2/M cell cycle arrest and apoptosis. Molecules 18:13200–13217
Liu S, Xu X, Ye J et al (2023) Metal-coordinated nanodrugs based on natural products for cancer theranostics. Chem Eng J 456:140892
Lu L-JW, Chen N-W, Brunder DG et al (2022) Soy isoflavones decrease fibroglandular breast tissue measured by magnetic resonance imaging in premenopausal women: a 2-year randomized double-blind placebo controlled clinical trial. Clinical Nutrition ESPEN 52:158–168
Luo Y, Wang S-x, Zhou Z-q et al (2014) Apoptotic effect of genistein on human colon cancer cells via inhibiting the nuclear factor-kappa B (NF-κB) pathway. Tumor Biology 35:11483–11488
Ma C-h, Zhang Y-x, Tang L-h et al (2018) MicroRNA-1469, a p53-responsive microRNA promotes genistein induced apoptosis by targeting Mcl1 in human laryngeal cancer cells. Biomed Pharmacother 106:665–671
Malojirao VH, Girimanchanaika SS, Shanmugam MK et al (2020) Novel 1, 3, 4-oxadiazole targets STAT3 signaling to induce antitumor effect in lung cancer. Biomedicines 8:368
Mesmar F, Dai B, Ibrahim A et al (2019) Clinical candidate and genistein analogue AXP107-11 has chemoenhancing functions in pancreatic adenocarcinoma through G protein-coupled estrogen receptor signaling. Cancer Med 8:7705–7719
Mittal P, Vrdhan H, Ajmal G, Bonde G, Kapoor R, Mishra B (2019) Formulation and characterization of genistein-loaded nanostructured lipid carriers: pharmacokinetic, biodistribution and in vitro cytotoxicity studies. Curr Drug Deliv 16:215–225
Mohammad RM, Al-Katib A, Aboukameel A, Doerge DR, Sarkar F, Kucuk O (2003) Genistein sensitizes diffuse large cell lymphoma to CHOP (cyclophosphamide, doxorubicin, vincristine, prednisone) chemotherapy. Mol Cancer Ther 2:1361–1368
Mohan CD, Bharathkumar H, Bulusu KC et al (2014) Development of a novel azaspirane that targets the Janus kinase-signal transducer and activator of transcription (STAT) pathway in hepatocellular carcinoma in vitro and in vivo. J Biol Chem 289:34296–34307
Mohan CD, Yang MH, Rangappa S et al (2021a) 3-Formylchromone counteracts STAT3 signaling pathway by elevating SHP-2 expression in hepatocellular carcinoma. Biology 11:29
Mohan CD, Rangappa S, Nayak SC, Sethi G, Rangappa KS (2021b) Paradoxical functions of long noncoding RNAs in modulating STAT3 signaling pathway in hepatocellular carcinoma. Biochimica et Biophysica Acta (BBA)-Reviews on Cancer 1876:188574
Mohan CD, Rangappa S, Preetham HD, et al (2022) Targeting STAT3 signaling pathway in cancer by agents derived from Mother Nature. Seminars in cancer biology. Elsevier, pp. 157–182
Naujokat C, McKee DL (2021) The “big five” phytochemicals targeting cancer stem cells: curcumin, EGCG, sulforaphane, resveratrol and genistein. Curr Med Chem 28:4321–4342
Nazim UM, Park S-Y (2015) Genistein enhances TRAIL-induced cancer cell death via inactivation of autophagic flux. Oncol Rep 34:2692–2698
Nozawa F, Itami A, Saruc M et al (2004) The combination of tumor necrosis factor–related apoptosis-inducing ligand (TRAIL/Apo2L) and genistein is effective in inhibiting pancreatic cancer growth. Pancreas 29:45–52
Obinu A, Burrai GP, Cavalli R et al (2021) Transmucosal solid lipid nanoparticles to improve genistein absorption via intestinal lymphatic transport. Pharmaceutics 13:267
Oishi M, Iizumi Y, Taniguchi T, Goi W, Miki T, Sakai T (2013) Apigenin sensitizes prostate cancer cells to Apo2L/TRAIL by targeting adenine nucleotide translocase-2. PLoS ONE 8:e55922
Ouyang G, Yao L, Ruan K, Song G, Mao Y, Bao S (2009) Genistein induces G2/M cell cycle arrest and apoptosis of human ovarian cancer cells via activation of DNA damage checkpoint pathways. Cell Biol Int 33:1237–1244
Parajuli B, Shin S-J, Kwon S-H et al (2013) The synergistic apoptotic interaction of indole-3-carbinol and genistein with TRAIL on endometrial cancer cells. J Korean Med Sci 28:527–533
Park C, Cha H-J, Lee H et al (2019) Induction of G2/M cell cycle arrest and apoptosis by genistein in human bladder cancer T24 cells through inhibition of the ROS-dependent PI3k/Akt signal transduction pathway. Antioxidants 8:327
Patra A, Satpathy S, Naik PK, Kazi M, Hussain MD (2022) Folate receptor-targeted PLGA-PEG nanoparticles for enhancing the activity of genistein in ovarian cancer. Artificial Cells, Nanomedicine, and Biotechnology 50:228–239
Paul B, Li Y, Tollefsbol TO (2018) The effects of combinatorial genistein and sulforaphane in breast tumor inhibition: role in epigenetic regulation. Int J Mol Sci 19:1754
Peffley DM, Sharma C, Hentosh P, Buechler RD (2007) Perillyl alcohol and genistein differentially regulate PKB/Akt and 4E-BP1 phosphorylation as well as eIF4E/eIF4G interactions in human tumor cells. Arch Biochem Biophys 465:266–273
Petak I, Houghton JA (2001) Shared pathways: death receptors and cytotoxic drugs in cancer therapy. Pathol Oncol Res 7:95–106
Pinski J, Wang Q, Quek ML et al (2006) Genistein-induced neuroendocrine differentiation of prostate cancer cells. Prostate 66:1136–1143
Pintova S, Dharmupari S, Moshier E, Zubizarreta N, Ang C, Holcombe RF (2019) Genistein combined with FOLFOX or FOLFOX–Bevacizumab for the treatment of metastatic colorectal cancer: phase I/II pilot study. Cancer Chemother Pharmacol 84:591–598
Pool H, Campos-Vega R, Herrera-Hernández MG et al (2018) Development of genistein-PEGylated silica hybrid nanomaterials with enhanced antioxidant and antiproliferative properties on HT29 human colon cancer cells. Am. J. Transl. Res. 10:2306
Qi W, Weber CR, Wasland K, Savkovic SD (2011) Genistein inhibits proliferation of colon cancer cells by attenuating a negative effect of epidermal growth factor on tumor suppressor FOXO3 activity. BMC Cancer 11:1–9
Qin J, Teng J, Zhu Z, Chen J, Huang W-J (2016) Genistein induces activation of the mitochondrial apoptosis pathway by inhibiting phosphorylation of Akt in colorectal cancer cells. Pharm Biol 54:74–79
Rasheed S, Rehman K, Shahid M, Suhail S, Akash MSH (2022) Therapeutic potentials of genistein: new insights and perspectives. J Food Biochem 46:e14228
Rendón JP, Cañas AI, Correa E et al (2022) Evaluation of the effects of genistein in vitro as a chemopreventive agent for colorectal cancer-strategy to improve its efficiency when administered orally. Molecules 27:7042
Sacko K, Thangavel K, Shoyele SA (2019) Codelivery of genistein and miRNA-29b to A549 cells using aptamer-hybrid nanoparticle bioconjugates. Nanomaterials 9:1052
Safa AR (2013) Roles of c-FLIP in apoptosis, necroptosis, and autophagy. Journal of carcinogenesis & mutagenesis
Sajith AM, Narasimhamurthy KH, Shanmugam MK et al (2021) Pyrimidine-2, 4-dione targets STAT3 signaling pathway to induce cytotoxicity in hepatocellular carcinoma cells. Bioorg Med Chem Lett 50:128332
Salem AM, Jackson IL, Gibbs A et al (2022) Interspecies comparison and radiation effect on pharmacokinetics of BIO 300, a nanosuspension of genistein, after different routes of administration in mice and non-human primates. Radiat Res 197:447–458
Schneider LS, Hernandez G, Zhao L et al (2019) Safety and feasibility of estrogen receptor β targeted phytoSERM formulation for menopausal symptoms: phase 1b/2a randomized clinical trial. Menopause (new York, NY) 26:874
Serebrenik AA, Verduyn CW, Kaytor MD (2023) Safety, pharmacokinetics, and biomarkers of an amorphous solid dispersion of genistein, a radioprotectant, in healthy volunteers. Clin. Pharmacol. Drug Dev. 12:190–201
Shafiee G, Saidijam M, Tavilani H, Ghasemkhani N, Khodadadi I (2016) Genistein induces apoptosis and inhibits proliferation of HT29 colon cancer cells. Int J Mol Cell Med 5:178
Shafiee G, Saidijam M, Tayebinia H, Khodadadi I (2022) Beneficial effects of genistein in suppression of proliferation, inhibition of metastasis, and induction of apoptosis in PC3 prostate cancer cells. Arch Physiol Biochem 128:694–702
Sharifi-Rad J, Quispe C, Mukazhanova Z et al (2021) Resveratrol-based nanoformulations as an emerging therapeutic strategy for cancer. Front Mol Biosci 8:649395
Sharma S, Malhotra L, Yadav P, Mishra V, Sharma RS, Samath EA (2022) Genistein: a novel inhibitor of IL-6/IL-6R interface of the interleukin-6–mediated STAT3 dependent pathway of carcinogenesis. J Mol Struct 1258:132668
Shen H, He D, Wang S, Ding P, Wang J, Ju J (2018) Preparation, characterization, and pharmacokinetics study of a novel genistein-loaded mixed micelles system. Drug Dev Ind Pharm 44:1536–1542
Shim H-Y, Park J-H, Paik H-D, Nah S-Y, Kim DS, Han YS (2007) Genistein-induced apoptosis of human breast cancer MCF-7 cells involves calpain-caspase and apoptosis signaling kinase 1–p38 mitogen-activated protein kinase activation cascades. Anticancer Drugs 18:649–657
Shukla RP, Dewangan J, Urandur S et al (2020) Multifunctional hybrid nanoconstructs facilitate intracellular localization of doxorubicin and genistein to enhance apoptotic and anti-angiogenic efficacy in breast adenocarcinoma. Biomaterials Science 8:1298–1315
Shushan A, Ben-Bassat H, Mishani E, Laufer N, Klein BY, Rojansky N (2007) Inhibition of leiomyoma cell proliferation in vitro by genistein and the protein tyrosine kinase inhibitor TKS050. Fertil Steril 87:127–135
Siegelin MD, Siegelin Y, Habel A, Gaiser T (2009) Genistein enhances proteasomal degradation of the short isoform of FLIP in malignant glioma cells and thereby augments TRAIL-mediated apoptosis. Neurosci Lett 453:92–97
Slee EA, Harte MT, Kluck RM et al (1999) Ordering the cytochrome c-initiated caspase cascade: hierarchical activation of caspases-2,-3,-6,-7,-8, and-10 in a caspase-9-dependent manner. J Cell Biol 144:281–292
Smeriglio A, Calderaro A, Denaro M, Laganà G, Bellocco E (2019) Effects of isolated isoflavones intake on health. Curr Med Chem 26:5094–5107
Sohel M, Biswas P, Al Amin M et al (2022) Genistein, a potential phytochemical against breast cancer treatment-insight into the molecular mechanisms. Processes 10:415
Soleimanpour M, Tamaddon AM, Kadivar M, Abolmaali SS, Shekarchizadeh H (2020) Fabrication of nanostructured mesoporous starch encapsulating soy-derived phytoestrogen (genistein) by well-tuned solvent exchange method. Int J Biol Macromol 159:1031–1047
Suksri K, Semprasert N, Limjindaporn T, Yenchitsomanus P-t, Kooptiwoot S, Kooptiwut S (2022) Cytoprotective effect of genistein against dexamethasone-induced pancreatic β-cell apoptosis. Sci Rep 12:12950
Sutrisno S, Aprina H, Simanungkalit HM et al (2018) Genistein modulates the estrogen receptor and suppresses angiogenesis and inflammation in the murine model of peritoneal endometriosis. Tradit. Complement. Med. 8:278–281
Szliszka E, Czuba ZP, Jernas K, Król W (2008) Dietary flavonoids sensitize HeLa cells to tumor necrosis factor-related apoptosis-inducing ligand (TRAIL). Int J Mol Sci 9:56–64
Tang Q, Ma J, Sun J et al (2018) Genistein and AG1024 synergistically increase the radiosensitivity of prostate cancer cells. Oncol Rep 40:579–588
Tian J, Guo F, Chen Y, Li Y, Yu B, Li Y (2019) Nanoliposomal formulation encapsulating celecoxib and genistein inhibiting COX-2 pathway and Glut-1 receptors to prevent prostate cancer cell proliferation. Cancer Lett 448:1–10
Tuli HS, Tuorkey MJ, Thakral F et al (2019) Molecular mechanisms of action of genistein in cancer: recent advances. Front Pharmacol 10:1336
Tuli HS, Joshi H, Vashishth K, et al (2023) Chemopreventive mechanisms of amentoflavone: recent trends and advancements. Naunyn-Schmiedeberg's Archives of Pharmacology
Vodnik VV, Mojić M, Stamenović U et al (2021) Development of genistein-loaded gold nanoparticles and their antitumor potential against prostate cancer cell lines. Mater Sci Eng, C 124:112078
Wang G, Zhang D, Yang S, Wang Y, Tang Z, Fu X (2018) Co-administration of genistein with doxorubicin-loaded polypeptide nanoparticles weakens the metastasis of malignant prostate cancer by amplifying oxidative damage. Biomater. Sci. 6:827–835
Wang Z, Yang L, Li Y et al (2022) An activatable, carrier-free, triple-combination nanomedicine for ALK/EGFR-mutant non-small cell lung cancer highly permeable targeted chemotherapy. New J Chem 46:17673–17677
Xu J, Loo G (2001) Different effects of genistein on molecular markers related to apoptosis in two phenotypically dissimilar breast cancer cell lines. J Cell Biochem 82:78–88
Xu Y, Zhang D, Yang H et al (2021) Hepatoprotective effect of genistein against dimethylnitrosamine-induced liver fibrosis in rats by regulating macrophage functional properties and inhibiting the JAK2/STAT3/SOCS3 signaling pathway. Frontiers in Bioscience-Landmark 26:1572–1584
Xu H, Ma H, Zha L, Li Q, Pan H, Zhang L (2022) Genistein promotes apoptosis of lung cancer cells through the IMPDH2/AKT1 pathway. Am. J. Transl. Res. 14:7040
Xue J-P, Wang G, Zhao Z-B, Wang Q, Shi Y (2014) Synergistic cytotoxic effect of genistein and doxorubicin on drug-resistant human breast cancer MCF-7/Adr cells. Oncol Rep 32:1647–1653
Yan H, Jiang J, Du A, Gao J, Zhang D, Song L (2020) Genistein enhances radiosensitivity of human hepatocellular carcinoma cells by inducing G2/M arrest and apoptosis. Radiat Res 193:286–300
Yang Y-Y, Tsai T-H (2019) Enterohepatic circulation and pharmacokinetics of genistin and genistein in rats. ACS Omega 4:18428–18433
Yang L, Wang Z (2021) Natural products, alone or in combination with FDA-approved drugs, to treat COVID-19 and lung cancer. Biomedicines 9:689
Yang Y, Zang A, Jia Y et al (2016a) Genistein inhibits A549 human lung cancer cell proliferation via miR-27a and MET signaling. Oncol Lett 12:2189–2193
Yang Y, Yang Y, Dai W, Li X, Ma J, Tang L (2016b) Genistein-induced apoptosis is mediated by endoplasmic reticulum stress in cervical cancer cells. Eur Rev Med Pharmacol Sci 20:3292–3296
Yang MH, Jung SH, Sethi G, Ahn KS (2019) Pleiotropic pharmacological actions of capsazepine, a synthetic analogue of capsaicin, against various cancers and inflammatory diseases. Molecules 24:995
Yeh T-C, Chiang P-C, Li T-K et al (2007) Genistein induces apoptosis in human hepatocellular carcinomas via interaction of endoplasmic reticulum stress and mitochondrial insult. Biochem Pharmacol 73:782–792
Yu L, Rios E, Castro L, Liu J, Yan Y, Dixon D (2021) Genistein: dual role in women’s health. Nutrients 13:3048
Zhang Q, Cao WS, Wang XQ et al (2019a) Genistein inhibits nasopharyngeal cancer stem cells through sonic hedgehog signaling. Phytother Res 33:2783–2791
Zhang Q, Bao J, Yang J (2019b) Genistein-triggered anticancer activity against liver cancer cell line HepG2 involves ROS generation, mitochondrial apoptosis, G2/M cell cycle arrest and inhibition of cell migrationand inhibition of cell migration. Arch Med Sci 15:1001–1009
Zhen AW, Nguyen NH, Gibert Y et al (2013) The small molecule, genistein, increases hepcidin expression in human hepatocytes. Hepatology 58:1315–1325
Zhou P, Wang C, Hu Z, Chen W, Qi W, Li A (2017) Genistein induces apoptosis of colon cancer cells by reversal of epithelial-to-mesenchymal via a Notch1/NF-κB/slug/E-cadherin pathway. BMC Cancer 17:1–10
Zhu Y, Zheng F, Xiao C, Liu X, Yao X, Zeng W (2022) Synthesis and bio-evaluation of 2-alkyl substituted fluorinated genistein analogues against breast cancer. Med Chem 18:589–601
Zou J-P, Zhang Z, Lv J-Y et al (2023) Design, synthesis and anti-cancer evaluation of genistein-1, 3, 5-triazine derivatives. Tetrahedron 134:133293
Author information
Authors and Affiliations
Contributions
HJ, HST, DSG, GK, NKA, CDM, JK, SR, IR, and DA conceived the conceptualization, methodology, validation, and writing, a review. MG and HSA performed the formal analysis and resources. HST did the data curation and editing. All authors have read and agreed to the published version of the manuscript. The authors declare that all data were generated in-house and that no paper mill was used.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
All authors have their consent to publish.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Joshi, H., Gupta, D.S., Abjani, N.K. et al. Genistein: a promising modulator of apoptosis and survival signaling in cancer. Naunyn-Schmiedeberg's Arch Pharmacol 396, 2893–2910 (2023). https://doi.org/10.1007/s00210-023-02550-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-023-02550-1