Abstract
Introduction
Genistein is recognized as a potent anti-oxidant in soybean-enriched foods, which is a kind of phytoestrogen involved in anticancer activity in various cancers.
Objective
The objective of this study was to investigate the molecular mechanism of CDKN2a hypomethylation involved in the anti-tumor effect of genistein on kidney cancer.
Methods
The CDKN2a expression was measured using qRT-PCR. The level of CDKN2a methylation was detected using methylation-specific PCR. The apoptosis was detected via flow-cytometric analysis. The cell viability was detected using the 3-(4, 5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay.
Results
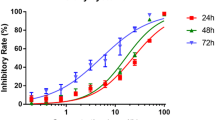
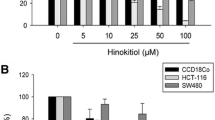
Our results indicated that genistein induced cell apoptosis and inhibited the cell proliferation of kidney cancer cells. Moreover, genistein increased the expression of CDKN2a and decreased CDKN2a methylation.
Conclusions
Our results demonstrated that the anti-tumor effect of genistein might induce cell apoptosis and inhibit the proliferation of kidney cancer cells via regulating CDKN2a methylation.






Similar content being viewed by others
References
Georgetti SR, Casagrande R, Vicentini FT, Baracat MM, Verri WA Jr, Fonseca MJ (2013) Protective effect of fermented soybean dried extracts against TPA-induced oxidative stress in hairless mice skin. Biomed Res Int 2013:340626
Ardito F, Di Gioia G, Pellegrino MR, Muzio LL (2018) Genistein as a potential anticancer agent against head and neck squamous cell carcinoma. Curr Top Med Chem 18:174–181
Jiang T, Wang XQ, Ding C, Du XL (2017) Genistein attenuates isoflurane-induced neurotoxicity and improves impaired spatial learning and memory by regulating cAMP/CREB and BDNF-TrkB-PI3K/Akt signaling. Korean J Physiol Pharmacol 21:579–589
Gong DK, Liu BH, Tan XH (2015) Genistein prevents cadmium-induced neurotoxic effects through its antioxidant mechanisms. Drug Res 65:65–69
Yang TT, Liu CG, Gao SC, Zhang Y, Wang PC (2018) The serum exosome derived MicroRNA-135a, -193b, and -384 were potential Alzheimer’s disease biomarkers. Biomed Environ Sci 31:87–96
Ahn SY, Jo MS, Lee D et al (2019) Dual effects of isoflavonoids from Pueraria lobata roots on estrogenic activity and anti-proliferation of MCF-7 human breast carcinoma cells. Bioorg Chem 83:135–144
Kamalakaran S, Varadan V, Giercksky Russnes HE et al (2011) DNA methylation patterns in luminal breast cancers differ from non-luminal subtypes and can identify relapse risk independent of other clinical variables. Mol Oncol 5:77–92
Brzezianska E, Dutkowska A, Antczak A (2013) The significance of epigenetic alterations in lung carcinogenesis. Mol Biol Rep 40:309–325
Caplakova V, Babusikova E, Blahovcova E, Balharek T, Zelieskova M, Hatok J (2016) DNA methylation machinery in the endometrium and endometrial cancer. Anticancer Res 36:4407–4420
Ma HL, Yu SJ, Chen J et al (2019) CA8 promotes RCC proliferation and migration though its expression level is lower in tumor compared to adjacent normal tissue. Biomed Pharmacother 121:109578
Udayakumar D, Mahato B, Gabree M, Tsao H (2010) Genetic determinants of cutaneous melanoma predisposition. Semin Cutan Med Surg 29:190–195
Freedberg DE, Rigas SH, Russak J et al (2008) Frequent p16-independent inactivation of p14ARF in human melanoma. J Natl Cancer Inst 100:784–795
Sherr CJ (2004) Principles of tumor suppression. Cell 116:235–246
Alhasan SA, Pietrasczkiwicz H, Alonso MD, Ensley J, Sarkar FH (1999) Genistein-induced cell cycle arrest and apoptosis in a head and neck squamous cell carcinoma cell line. Nutr Cancer 34:12–19
Nosaka K, Maeda M, Tamiya S, Sakai T, Mitsuya H, Matsuoka M (2000) Increasing methylation of the CDKN2A gene is associated with the progression of adult T-cell leukemia. Can Res 60:1043–1048
Tam KW, Zhang W, Soh J et al (2013) CDKN2A/p16 inactivation mechanisms and their relationship to smoke exposure and molecular features in non-small-cell lung cancer. J Thorac Oncol 8:1378–1388
Pierini S, Jordanov SH, Mitkova AV et al (2014) Promoter hypermethylation of CDKN2A, MGMT, MLH1, and DAPK genes in laryngeal squamous cell carcinoma and their associations with clinical profiles of the patients. Head Neck 36:1103–1108
Csepregi A, Ebert MP, Rocken C et al (2010) Promoter methylation of CDKN2A and lack of p16 expression characterize patients with hepatocellular carcinoma. BMC Cancer 10:317
Sinha S, Chunder N, Mukherjee N et al (2008) Frequent deletion and methylation in SH3GL2 and CDKN2A loci are associated with early- and late-onset breast carcinoma. Ann Surg Oncol 15:1070–1080
Ito S, Ohga T, Saeki H et al (2007) Promoter hypermethylation and quantitative expression analysis of CDKN2A (p14ARF and p16INK4a) gene in esophageal squamous cell carcinoma. Anticancer Res 27:3345–3353
Yang J, Bai WL, Chen YJ, Gao A (2015) 1,4-benzoquinone-induced STAT-3 hypomethylation in AHH-1 cells: role of oxidative stress. Toxicol Rep 2:864–869
Baxa DM, Luo X, Yoshimura FK (2005) Genistein induces apoptosis in T lymphoma cells via mitochondrial damage. Nutr Cancer 51:93–101
Spagnuolo C, Russo GL, Orhan IE et al (2015) Genistein and cancer: current status, challenges, and future directions. Adv Nutr 6:408–419
Tang Q, Ma J, Sun J et al (2018) Genistein and AG1024 synergistically increase the radiosensitivity of prostate cancer cells. Oncol Rep 40(2):579–588
Al-Maghrebi M, Renno WM (2016) Genistein alleviates testicular ischemia and reperfusion injury-induced spermatogenic damage and oxidative stress by suppressing abnormal testicular matrix metalloproteinase system via the Notch 2/Jagged 1/Hes-1 and caspase-8 pathways. J Physiol Pharmacol 67:129–137
Stenvinkel P, Luttropp K, McGuinness D et al (2017) CDKN2A/p16INK4(a) expression is associated with vascular progeria in chronic kidney disease. Aging 9:494–507
Liu K, Zhao C, Chen J et al (2016) Overexpression of SEPP1 inhibits the proliferation and induces cell cycle G2/M arrest of 786-O and 769-P human renal carcinoma cells. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 32:764–769
Pan T, Fong EL, Martinez M et al (2015) Three-dimensional (3D) culture of bone-derived human 786-O renal cell carcinoma retains relevant clinical characteristics of bone metastases. Cancer Lett 365:89–95
Ricketts CJ, Morris MR, Gentle D et al (2013) Methylation profiling and evaluation of demethylating therapy in renal cell carcinoma. Clin Epigenet 5:16
Deng D, Liu Z, Du Y (2010) Epigenetic alterations as cancer diagnostic, prognostic, and predictive biomarkers. Adv Genet 71:125–176
Lasseigne BN, Brooks JD (2018) The role of DNA methylation in renal cell carcinoma. Mol Diagn Ther 22(4):431–442
Kanai Y (2010) Genome-wide DNA methylation profiles in precancerous conditions and cancers. Cancer Sci 101:36–45
Al-Saran N, Subash-Babu P, Al-Nouri DM, Alfawaz HA, Alshatwi AA (2016) Zinc enhances CDKN2A, pRb1 expression and regulates functional apoptosis via upregulation of p53 and p21 expression in human breast cancer MCF-7 cell. Environ Toxicol Pharmacol 47:19–27
Hui KF, Leung YY, Yeung PL, Middeldorp JM, Chiang AK (2014) Combination of SAHA and bortezomib up-regulates CDKN2A and CDKN1A and induces apoptosis of Epstein-Barr virus-positive Wp-restricted Burkitt lymphoma and lymphoblastoid cell lines. Br J Haematol 167:639–650
Gossner G, Choi M, Tan L et al (2007) Genistein-induced apoptosis and autophagocytosis in ovarian cancer cells. Gynecol Oncol 105:23–30
Shafiee G, Saidijam M, Tavilani H, Ghasemkhani N, Khodadadi I (2016) Genistein induces apoptosis and inhibits proliferation of HT29 colon cancer cells. Int J Mol Cell Med 5:178–191
Chen HH, Chen SP, Zheng QL et al (2018) Genistein promotes proliferation of human cervical cancer cells through estrogen receptor-mediated PI3K/Akt-NF-kappaB pathway. J Cancer 9:288–295
Yang YM, Yang Y, Dai WW, Li XM, Ma JQ, Tang LP (2016) Genistein-induced apoptosis is mediated by endoplasmic reticulum stress in cervical cancer cells. Eur Rev Med Pharmacol Sci 20:3292–3296
The Cancer Genome Atlas Research Network (2016) Comprehensive molecular characterization of papillary renal-cell carcinoma. N Engl J Med 374:135–145
Acknowledgements
Our study has been supported by grants from Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special Funding Support (ZYLX201604). We thank Dr. X. Qing for providing technical support.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
None of the authors has any commercial or other associations that might pose a conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ji, Z., Huo, C. & Yang, P. Genistein inhibited the proliferation of kidney cancer cells via CDKN2a hypomethylation: role of abnormal apoptosis. Int Urol Nephrol 52, 1049–1055 (2020). https://doi.org/10.1007/s11255-019-02372-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-019-02372-2