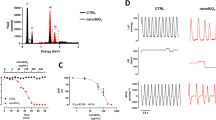
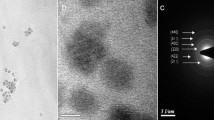
Abstract
Superparamagnetic iron oxide nanoparticles (IONPs) have been widely applied in numerous biomedical fields. The evaluation of the toxicity of IONPs to the environment and human beings is indispensable to guide their applications. IONPs are usually considered to have good biocompatibility; however, some literatures have reported the toxicity of IONPs in vitro and in vivo. The controversy surrounding the biocompatibility of IONPs prompted us to carefully consider the biological effects of IONPs, especially under stress conditions. However, the potential risks of IONPs under stress conditions have not yet been evaluated in depth. Acrolein is widespread in the environment and modulates stress-induced gene activation and cell death in many organs and tissues. In this study, we assessed the sensitivity of H9c2 cardiomyocyte cells embedded with IONPs to acrolein and investigated the possible molecular mechanisms involved in this sensitivity. IONPs, which alone exhibited no toxicity, sensitized the H9c2 cardiomyocytes to acrolein-induced dysfunction. The IONP/acrolein treatment induced a loss of viability, membrane disruption, reactive oxygen species (ROS) generation, Erk activation, mitochondrial and lysosomal dysfunction, and necrosis in H9c2 cells. Treatment with an ROS generation inhibitor (diphenyleneiodonium) or an iron chelator (deferoxamine) prevented the IONP/acrolein-induced loss of viability, suggesting that ROS and IONP degradation facilitated the toxicity of the IONP/acrolein treatment in H9c2 cells. Our data suggest that cells embedded in IONPs are more vulnerable to oxidative stress, which confirms the hypothesis that nanoparticles can sensitize cells to the adverse effects of external stimulation. The present work provides a new perspective from which to evaluate the interactions between nanoparticles and cells.






Similar content being viewed by others
Abbreviations
- AO:
-
Acridine orange
- ATP:
-
Adenosine 5′-triphosphate
- BCA:
-
Bicinchoninic acid assay
- BSA:
-
Bovine serum albumin
- DHE:
-
Dihydroethidium
- DLS:
-
Dynamic light scattering
- DMEM:
-
Dulbecco’s modified eagle medium
- DNPH:
-
2,4-Dinitrophenylhydrazine
- DPI:
-
Diphenyleneiodonium
- EGF:
-
Epidermal growth factor
- Erk:
-
Extracellular signal-regulated kinase
- ESCs:
-
Embryonic stem cells
- FBS:
-
Fetal bovine serum
- GSH:
-
Glutathione reduced
- HCM:
-
Human cardiac myocytes
- IONPs:
-
Superparamagnetic iron oxide nanoparticles
- JC-1:
-
CBIC2(3), 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetra ethylbenzimidazolylcarbocyanine iodide
- JNK:
-
c-Jun N-terminal kinase
- LAMP1:
-
Lysosome-associated membrane protein 1
- LDH:
-
Lactate dehydrogenase
- MDC:
-
Monodansylcadaverine
- MTT:
-
3-(4,5)-Dimethylthiahiazo (-z-y1)-3,5-di-phenytetrazoliumromide
- MRI:
-
Magnetic resonance imaging
- NDA:
-
2,3-Naphthalenedicarboxaldehyde
- NOX:
-
Nicotinamide adenine dinucleotide phosphate oxidase
- PBS:
-
Phosphate-buffered saline
- PI:
-
Propidium iodide
- RIP:
-
Receptor-interacting protein
- ROS:
-
Reactive oxygen species
- tBHP:
-
Tert-butyl hydroperoxide
- TEM:
-
Transmission electron microscope
References
Anderson EJ, Katunga LA, Willis MS (2012) Mitochondria as a source and target of lipid peroxidation products in healthy and diseased heart. Clin Exp Pharmacol Physiol 39(2):179–193
Andreas K, Georgieva R, Ladwig M, Mueller S, Notter M, Sittinger M, Ringe J (2012) Highly efficient magnetic stem cell labeling with citrate-coated superparamagnetic iron oxide nanoparticles for MRI tracking. Biomaterials 33(18):4515–4525
Ansari MA, Keller JN, Scheff SW (2008) Protective effect of pycnogenol in human neuroblastoma SH-SY5Y cells following acrolein-induced cytotoxicity. Free Radic Biol Med 45(11):1510–1519
Au KW, Liao SY, Lee YK, Lai WH, Ng KM, Chan YC, Yip MC, Ho CY, Wu EX, Li RA, Siu CW, Tse HF (2009) Effects of iron oxide nanoparticles on cardiac differentiation of embryonic stem cells. Biochem Biophys Res Commun 379(4):898–903
Bajaj A, Samanta B, Yan H, Jerry DJ, Rotello VM (2009) Stability, toxicity and differential cellular uptake of protein passivated-Fe3O4 nanoparticles. J Mater Chem 19(35):6328–6331
Bein K, Leikauf GD (2011) Acrolein—a pulmonary hazard. Mol Nutr Food Res 55(9):1342–1360
Boya P, Kroemer G (2008) Lysosomal membrane permeabilization in cell death. Oncogene 27(50):6434–6451
Chen YC, Hsiao JK, Liu HM, Lai IY, Yao M, Hsu SC, Ko BS, Yang CS, Huang DM (2010) The inhibitory effect of superparamagnetic iron oxide nanoparticle (Ferucarbotran) on osteogenic differentiation and its signaling mechanism in human mesenchymal stem cells. Toxicol Appl Pharmacol 245(2):272–279
Chen Z, Yin JJ, Zhou YT, Zhang Y, Song L, Song M, Hu S, Gu N (2012) Dual enzyme-like activities of iron oxide nanoparticles and their implication for diminishing cytotoxicity. ACS Nano 6(5):4001–4012
Colombo M, Carregal-Romero S, Casula MF, Gutierrez L, Morales MP, Bohm IB, Heverhagen JT, Prosperi D, Parak WJ (2012) Biological applications of magnetic nanoparticles. Chem Soc Rev 41(11):4306–4334
Comfort KK, Maurer EI, Braydich-Stolle LK, Hussain SM (2011) Interference of silver, gold, and iron oxide nanoparticles on epidermal growth factor signal transduction in epithelial cells. ACS Nano 5(12):10000–10008
Feng Z, Hu W, Hu Y, Tang MS (2006) Acrolein is a major cigarette-related lung cancer agent: preferential binding at p53 mutational hotspots and inhibition of DNA repair. Proc Natl Acad Sci U S A 103(42):15404–15409
Huang G, Chen H, Dong Y, Luo X, Yu H, Moore Z, Bey EA, Boothman DA, Gao J (2013) Superparamagnetic iron oxide nanoparticles: amplifying ROS stress to improve anticancer drug efficacy. Theranostics 3(2):116–126
Kehrer JP (2000) The Haber–Weiss reaction and mechanisms of toxicity. Toxicology 149(1):43–50
Khan MI, Mohammad A, Patil G, Naqvi SA, Chauhan LK, Ahmad I (2012) Induction of ROS, mitochondrial damage and autophagy in lung epithelial cancer cells by iron oxide nanoparticles. Biomaterials 33(5):1477–1488
Kung G, Konstantinidis K, Kitsis RN (2011) Programmed necrosis, not apoptosis, in the heart. Circ Res 108(8):1017–1036
Kurz T, Gustafsson B, Brunk UT (2006) Intralysosomal iron chelation protects against oxidative stress-induced cellular damage. FEBS J 273(13):3106–3117
Lee CH, Hung HW, Hung PH, Shieh YS (2010) Epidermal growth factor receptor regulates beta-catenin location, stability, and transcriptional activity in oral cancer. Mol Cancer 9:64
Levy M, Lagarde F, Maraloiu VA, Blanchin MG, Gendron F, Wilhelm C, Gazeau F (2010) Degradability of superparamagnetic nanoparticles in a model of intracellular environment: follow-up of magnetic, structural and chemical properties. Nanotechnology 21(39):395103
Li Q, Tang G, Xue S, He X, Miao P, Li Y, Wang J, Xiong L, Wang Y, Zhang C, Yang G-Y (2013) Silica-coated superparamagnetic iron oxide nanoparticles targeting of EPCs in ischemic brain injury. Biomaterials 34(21):4982–4992
Liu G, Gao J, Ai H, Chen X (2013) Applications and potential toxicity of magnetic iron oxide nanoparticles. Small 9(9–10):1533–1545
Lunov O, Syrovets T, Buchele B, Jiang X, Rocker C, Tron K, Nienhaus GU, Walther P, Mailander V, Landfester K, Simmet T (2010) The effect of carboxydextran-coated superparamagnetic iron oxide nanoparticles on c-Jun N-terminal kinase-mediated apoptosis in human macrophages. Biomaterials 31(19):5063–5071
Luo C, Li Y, Wang H, Cui Y, Feng Z, Li H, Li Y, Wang Y, Wurtz K, Weber P, Long J, Liu J (2013a) Hydroxytyrosol promotes superoxide production and defects in autophagy leading to anti-proliferation and apoptosis on human prostate cancer cells. Curr Cancer Drug Targets 13(6):625–639
Luo C, Li Y, Wang H, Feng Z, Long J, Liu J (2013b) Mitochondrial accumulation under oxidative stress is due to defects in autophagy. J Cell Biochem 114(1):212–219
Luo C, Wang H, Chen X, Cui Y, Li H, Long J, Mo X, Liu J (2013c) Protection of H9c2 rat cardiomyoblasts against oxidative insults by total paeony glucosides from radix paeoniae rubrae. Phytomedicine 21(1):20–24
Maghzal GJ, Krause KH, Stocker R, Jaquet V (2012) Detection of reactive oxygen species derived from the family of NOX NADPH oxidases. Free Radic Biol Med 53(10):1903–1918
Mahmoudi M, Laurent S, Shokrgozar MA, Hosseinkhani M (2011) Toxicity evaluations of superparamagnetic iron oxide nanoparticles: cell “vision” versus physicochemical properties of nanoparticles. ACS Nano 5(9):7263–7276
Mahmoudi M, Hofmann H, Rothen-Rutishauser B, Petri-Fink A (2012) Assessing the in vitro and in vivo toxicity of superparamagnetic iron oxide nanoparticles. Chem Rev 112(4):2323–2338
Mohammad MK, Avila D, Zhang J, Barve S, Arteel G, McClain C, Joshi-Barve S (2012) Acrolein cytotoxicity in hepatocytes involves endoplasmic reticulum stress, mitochondrial dysfunction and oxidative stress. Toxicol Appl Pharmacol 265(1):73–82
Park EJ, Kim H, Kim Y, Yi J, Choi K, Park K (2010) Inflammatory responses may be induced by a single intratracheal instillation of iron nanoparticles in mice. Toxicology 275(1–3):65–71
Ramesh V, Ravichandran P, Copeland CL, Gopikrishnan R, Biradar S, Goornavar V, Ramesh GT, Hall JC (2012) Magnetite induces oxidative stress and apoptosis in lung epithelial cells. Mol Cell Biochem 363(1–2):225–234
Rauch J, Kolch W, Laurent S, Mahmoudi M (2013) Big signals from small particles: regulation of cell signaling pathways by nanoparticles. Chem Rev 113(5):3391–3406
Saito S, Tsugeno M, Koto D, Mori Y, Yoshioka Y, Nohara S, Murase K (2012) Impact of surface coating and particle size on the uptake of small and ultrasmall superparamagnetic iron oxide nanoparticles by macrophages. Int J Nanomedicine 7:5415–5421
Shi R, Rickett T, Sun W (2011) Acrolein-mediated injury in nervous system trauma and diseases. Mol Nutr Food Res 55(9):1320–1331
Singh N, Jenkins GJ, Asadi R, Doak SH (2010) Potential toxicity of superparamagnetic iron oxide nanoparticles (SPION). Nano Rev 1. doi:10.3402/nano.v1i0.5358, http://www.nano-reviews.net/index.php/nano/article/view/5358/6032
Singh N, Jenkins GJ, Nelson BC, Marquis BJ, Maffeis TG, Brown AP, Williams PM, Wright CJ, Doak SH (2012) The role of iron redox state in the genotoxicity of ultrafine superparamagnetic iron oxide nanoparticles. Biomaterials 33(1):163–170
Soenen SJ, Himmelreich U, Nuytten N, Pisanic TR 2nd, Ferrari A, De Cuyper M (2010) Intracellular nanoparticle coating stability determines nanoparticle diagnostics efficacy and cell functionality. Small 6(19):2136–2145
Soenen SJ, Himmelreich U, Nuytten N, De Cuyper M (2011) Cytotoxic effects of iron oxide nanoparticles and implications for safety in cell labelling. Biomaterials 32(1):195–205
Stevens JF, Maier CS (2008) Acrolein: sources, metabolism, and biomolecular interactions relevant to human health and disease. Mol Nutr Food Res 52(1):7–25
Stroh A, Zimmer C, Gutzeit C, Jakstadt M, Marschinke F, Jung T, Pilgrimm H, Grune T (2004) Iron oxide particles for molecular magnetic resonance imaging cause transient oxidative stress in rat macrophages. Free Radic Biol Med 36(8):976–984
Subramaniam S, Unsicker K (2006) Extracellular signal-regulated kinase as an inducer of non-apoptotic neuronal death. Neuroscience 138(4):1055–1065
Sun Z, Yathindranath V, Worden M, Thliveris JA, Chu S, Parkinson FE, Hegmann T, Miller DW (2013) Characterization of cellular uptake and toxicity of aminosilane-coated iron oxide nanoparticles with different charges in central nervous system-relevant cell culture models. Int J Nanomedicine 8:961–970
Yu MK, Jeong YY, Park J, Park S, Kim JW, Min JJ, Kim K, Jon S (2008) Drug-loaded superparamagnetic iron oxide nanoparticles for combined cancer imaging and therapy in vivo. Angew Chem Int Ed 47(29):5362–5365
Acknowledgments
This study was partially supported by the National Natural Science Foundation of China, Key Program 30930105, Foundation of Xi’an Jiaotong University, New Century Excellent Talents in University, the National Natural Science Foundation of China (Grant No. 31070740), and the 985 and 211 Projects of Xi’an Jiaotong University.
Conflict of interest
The authors confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Luo, C., Li, Y., Yang, L. et al. Superparamagnetic iron oxide nanoparticles exacerbate the risks of reactive oxygen species-mediated external stresses. Arch Toxicol 89, 357–369 (2015). https://doi.org/10.1007/s00204-014-1267-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-014-1267-x