Abstract
Background
During the recent pandemic with the severe acute respiratory syndrome-corona virus‑2 the first messenger ribonucleic acid (mRNA) vaccines were approved. To facilitate mass vaccination, confidence of the general population in these new vaccines is mandatory, which is in turn strongly dependent on the availability of reliable data on complications.
Objective
Summary of the current knowledge on mRNA vaccination-associated myocarditis as a potentially fatal side effect.
Methods
Systematic literature review.
Results
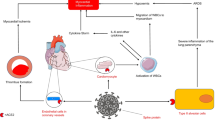
Diagnostic algorithm for the postmortem diagnosis of mRNA vaccination-associated myocarditis.
Conclusion
Autopsy series of fatalities following mRNA SARS-CoV‑2 vaccination up to 6 weeks with subsequent sophisticated and interdisciplinary work-up are necessary to complement clinical data on vaccination-associated myocarditis, especially regarding the incidence of fatal courses.
Zusammenfassung
Hintergrund
Mit der aktuellen Pandemie mit dem severe acute respiratory syndrome-corona virus‑2 (SARS-CoV‑2) wurden die ersten messenger Ribonukleinsäure (mRNA)-Impfstoffe zugelassen. Der Erfolg der Impfkampagne hängt vom Vertrauen der Bevölkerung in die neuen Impfstoffe, und dieses wiederum von der Verfügbarkeit reliabler Daten zu Komplikationen ab.
Ziel der Arbeit
Zusammenfassung des aktuellen Kenntnisstandes zu impfassoziierten Myokarditiden, als potenziell letalen Nebenwirkungen der mRNA-Impfstoffe.
Methoden
Systematischer Literatur-Review.
Ergebnisse
Diagnostischer Algorithmus zur systematischen postmortalen Aufarbeitung von Todesfällen im zeitlichen Zusammenhang mit einer mRNA-Impfung hinsichtlich einer impfassoziierten Myokarditis.
Diskussion
Die impfassoziierte Myokarditis ist eine Ausschlussdiagnose, die lediglich durch eine differenzierte und interdisziplinäre Aufarbeitung gestellt werden kann. Autopsieserien von Todesfällen bis zu 6 Wochen nach der Impfung sind erforderlich, um die klinischen Daten hinsichtlich letaler Komplikationen zu ergänzen.
Similar content being viewed by others
Introduction
Effective forms of treatment for the coronavirus disease 2019 (COVID-19) are limited [1]. Thus, mass vaccination is applied to protect individuals from serious disease and gain control of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic [2]. New virus variants can exhibit increased virulence [3] and drive breakthrough COVID-19 cases despite full immunization [4]. Nevertheless, vaccination is effective against several virus variants [5, 6] and protects against severe disease in the case of a breakthrough [7]. How often adjustments of the vaccines and boosters will be necessary is currently unclear. Thus, it seems likely that vaccination remains a dominating control strategy. The pandemic led to the first approval of messenger ribonucleic acid (mRNA) vaccines [8], from the scientific point of view a well and decade-long known [9] promising technology [8] with a wide range of potential applications far beyond SARS-CoV‑2 [8, 9]. Nevertheless, from the general population’s point of view it remains a “new” technology having been rapidly approved for the first time. As new vaccines are sometimes confronted with public skepticism [10], reliable data about their safety are the basis for general acceptance [11]. Thus, acquisition of reliable data regarding complications of mRNA vaccines is mandatory to support mass vaccination.
The mRNA vaccination-associated myocarditis (VAM) was repeatedly discussed in the media. Clinical studies showed that the mRNA-VAM usually has a mild course [12]. Nevertheless, a myocarditis is potentially fatal [13, 14]. Systematic postmortem work-up of fatalities after mRNA vaccination would be the basis for reliable epidemiological data regarding fatal side effects. Such data are hardly available (e.g. [15]), and do not comprise a systematic diagnostic work-up [15].
This systematic review of clinical case reports on mRNA-VAM collects data on the typical diagnostic findings and potential, or necessary differential diagnostic steps required for reliable diagnosis of VAM. As a result of this review, a first, basic postmortem diagnostic algorithm for the VAM is presented.
Methods
Systematic literature research using the National Library of Medicine database (https://pubmed.ncbi.nlm.nih.gov/) was conducted (advanced search algorithm: “(vaccine) AND (myocarditis)”; inclusion and exclusion criteria in Table 1). According to the first approval of a mRNA vaccine, articles from December 2020 onwards were included. The last database query was performed on 1 September 2021. Only cases of SARS-CoV‑2 mRNA-VAM were included in this review. Calculations were performed using Microsoft® (Redmond, WA, USA) Excel 2016.
Results
The algorithm identified 126 articles, of which 25 (19.8%) met the inclusion criteria. These articles describe 66 cases of mRNA-VAM. BNT162b2 (manufacturer: Pfizer® [New York City, NY, USA]-BioNTech® [Mainz, Germany], also known as Tozinameran or Comirnaty [16]) was administered in 53 cases (80.3%) and mRNA-1273 (manufacturer: Moderna® [Cambridge, MA, USA], also known as Elasomeran or Spikevax [16]) in 13 cases (19.7%).
In 57 (86.4%) cases, myocarditis occurred following the second vaccination. In 51 instances no previous illness was reported while in 6 instances analysis of the medical history yielded prior illness:
-
I.
17-year-old male, status post-myocarditis in 2014 (vaccine: BNT162b2, [17])
-
II.
15-year-old boy with obesity and insulin resistance (vaccine: BNT162b2, [17])
-
III.
67-year-old male with type 2 diabetes, arterial hypertension, heart failure with preserved ejection fraction, status post-multiple coronary interventions, and chronic obstructive pulmonary disease (vaccine: mRNA-1273, [18])
-
IV.
39-year-old male with autoimmune hypothyroidism, and status post-spontaneous pneumothorax and lobectomy (vaccine: BNT162b2, [19])
-
V.
70-year-old female with arterial hypertension (vaccine: mRNA-1273, [20])
-
VI.
52-year-old male with multiple diseases, including arterial hypertension, steatosis hepatis, and coronary calcification (vaccine: mRNA-1273, [21]).
After the first dose myocarditis occurred in 9 (13.6%) cases [17, 22,23,24,25,26,27,28], with 2 of these individuals having pre-existing conditions:
-
I.
15-year-old male with Marfan syndrome (vaccine: BNT162b2, [17])
-
II.
21-year-old male with bronchial asthma and allergies (vaccine: BNT162b2, [24])
In 7 instances (10.6%), a history of SARS-CoV‑2 infection prior to vaccination was reported (in 1 case mRNA-1273 vaccine [22]; in 6 cases BNT162b2 vaccine [22, 25, 26, 29]). Myocarditis occurred after the first dose in 4 of these cases [17, 22, 26]. In 2 of the 66 cases (3%), no information on previous SARS-CoV‑2 infection was available [30, 31].
More males (62 of 66 cases; 93.9%), than females (4 of 66; 6.1% [20, 22, 30]) were affected. In 3 of the women, mRNA-1273 was administered [20, 22, 30]. The average age of affected women was 34.8 years (range: 17–70 years), and for affected men 24.5 years (range: 14–67 years, average age of men affected after first dose 25.1 years; average age of men affected after second dose 24.4 years).
Imaging
Magnetic resonance imaging (MRI) was reported in 57 cases (86.4%; 9 cases (13.6%) without reported MRI [17, 18, 28, 32]). For MRI-based diagnosis usually the Lake Louise Criteria were applied (e.g. [33]). Late gadolinium enhancement was typically located subepicardially (e.g. [33]). If no MRI was reported, diagnosis was based on laboratory findings, clinical presentation, and electrocardiogram (ECG) changes [32].
The left ventricular ejection fraction (LVEF) was reported in all but 1 case (98.5% of the included cases). In 60 cases (90.9%), LVEF was described as “normal”, “good”, “preserved” or “more than 50%”. In 6 cases (9.1%), LVEF was below 50% [20, 25, 26, 33, 34].
Endomyocardial biopsy
Endomyocardial biopsies were reported in 2 cases (3%), without further information on number and localization of the biopsies [25, 26]. In both instances, no histological evidence of myocarditis was found [25, 26].
Differential diagnostic work-up: infectiology
In none of the cases virological analysis of myocardium was reported while other virological work-up to a varying extent was reported in 20 cases (30.3%). In 2 cases (3%) only influenza testing was mentioned [32]. Other case reports described virus panels that were not further explained, such as a “respiratory virus panel” [25] or a “cardiotropic virus panel” [31].
Some reports described varying types of bacteriological work-up [33]. Blood cultures were acquired in 1 case (1.5%) [34]. Mycoplasma pneumonia was found in 1 case (1.5%) [35], and this individual was prophylactically treated with doxycycline [35]. In 1 instance (1.5%), unspecified treatment of sepsis was reported [18].
Differential diagnostic work-up: autoimmune disorders
In 8 cases (12.1%), autoimmune disease work-up was explicitly mentioned [21, 27, 36]. In 1 report, antibody and immune cell analyses were performed [21], while other reports did not specify their autoimmune diagnostic panel further [27, 36]. None of the analyzed cases exhibited an autoimmune disorder [21, 27, 36].
Differential diagnostic work-up: toxic myocarditis
In 6 cases (9.1%), toxic myocarditis was explicitly mentioned as a differential diagnosis. In these, the medical history yielded no hint for toxic myocarditis [27].
The supplement provides more details on identified clinical reports (further reading) and clinical features and the time to onset of symptoms after vaccination.
Discussion
Epidemiology of myocarditis
The prevalence of myocarditis in the general population has been reported between 10 and 105 individuals per 100,000 [37]. Viruses have been described as the most frequent cause of myocarditis [14], with 1–10 viral myocarditis cases per 100,000 per year [38]. Globally, the coxsackie virus is the most frequently detected virus [37].
SARS-CoV‑2 can cause acute and chronic myocardial damage [39]. Of 100,000 infected patients 1000–4000 have been estimated to develop a COVID-19-associated myocarditis [38]. Compared to these numbers, mRNA SARS-CoV-2-VAM is rare with around 2.13 cases out of 100,000 with the highest incidence in the young (i.e. between 16 and 29 years) [40]. Survival rates of more than 99% have been described for the mRNA-VAM [38], although no systematic work-up of fatalities in chronological connection to mRNA SARS-CoV‑2 immunization has been presented to date. Thus, unknown fatalities due to mRNA-VAM must be assumed.
Assessing the reviewed cases
According to diagnostic criteria developed for smallpox VAM, diagnosis of VAM can only be confirmed by histology [41], while diagnosis based on MRI and laboratory tests is classified as probable [41]. Diagnoses based on ECG and clinical appearance alone are rated as suspected cases [41]. Thus, applying the aforementioned diagnostic criteria, none of the 66 cases reviewed can be rated as confirmed VAM. In 57 cases, the diagnosis is probable, and in 9 instances suspected.
Endomyocardial biopsies and MRI
Myocarditis is a “patchy” pathology [42] somehow scattered over the heart. Therefore, up to 17 endomyocardial biopsies have been reported to be required to detect approximately 79% of cases of right ventricular involvement [43]. In the postmortem setting at least 16 samples (i.e. 8 left, and 8 right ventricular specimens, including the conduction system) are recommended [44].
Since these diagnostic criteria were developed, various diagnostic modalities have improved. Nowadays, MRI using the Lake Louise criteria allows diagnosis with a sensitivity of 69% and a specificity of 91% [45]. So, cardiac MRI might be the most important diagnostic tool in a clinical setting [46], although in early stages of inflammation MRI is restricted [47]. In the postmortem setting MRI is not yet elaborated for the diagnosis of myocarditis [48].
The mRNA SARS-CoV‑2 VAM inflammatory lesions are usually positioned subepicardial (e.g. [34]). Thus, reaching them by endomyocardial biopsy without jeopardizing the patients’ well-being is questionable. Consequently, the diagnostic criteria developed for the smallpox VAM are hardly applicable nowadays for the diagnosis of mRNA-VAM.
Histology and myocarditis
Histological diagnosis of myocarditis is limited by numerous factors, including interobserver variability [49], sampling error [50, 51], and the broad etiological [14, 37] and histological spectrum of myocarditis [13]. Hence, a high level of expertise is necessary for sufficient histological diagnosis of myocarditis [50] (e.g. differentiation of distinct eosinophilic heart syndromes [52]). Nevertheless, histology, especially immunohistology, is mandatory for the diagnosis of myocarditis [44, 53, 54]. The importance of histology in the postmortem setting is strengthened by postmortem limitations of MRI [48] and an almost unrestricted access to myocardial tissue during autopsy. Thus, histology is the key to the diagnosis of myocarditis in the (forensic) pathological setting allowing identification of predisposed areas, characterization of the inflammatory lesions, and detection of small (potentially early and subclinical) lesions.
Differentiation of the cell types forming the infiltrates (e.g. by immunohistochemistry [55]) and molecular pathology [56] may provide insights into the mechanisms underlying mRNA SARS-CoV‑2 VAM and potentially allow differentiation between “real” mRNA-VAM and other simply coincident myocarditis cases due to a more likely cause such as a virus [14].
Differential diagnosis
Causes of myocarditis are manifold (e.g. several drugs [37], toxins [57, 58], hypersensitive/autoimmune phenomena [37]). Thus, to determine whether a myocarditis is just a random coincidence with the mRNA SARS-CoV-2-immunization or is a “real” VAM, requires differential work-up. Thereby, VAM is a diagnosis of exclusion. So, more frequent and thus more likely causes of the myocarditis must be sufficiently ruled out. Hence, also in the postmortem setting, especially the viral myocarditis mut be ruled out during the diagnostic process of VAM. Accordingly, complementary to histological specimens, blood samples and myocardial samples for virological work-up should be obtained [53] and analyzed as soon as histology confirms myocarditis. Also, depending on regional conditions and in adjustment to the respective case, samples allowing further toxicological, rheumatological, and other work-up should be obtained.
Reports of individuals with immanent myocarditis and conduction disturbances after the vaccination are available [59]. Whether this is a distinct entity, or a subform of subclinical myocarditis is not yet known, therefore postmortem work-up of fatalities in a chronological connection with a mRNA-SARS-CoV-2-immunization is necessary.
mRNA SARS-CoV-2 VAM
The reviewed case reports suggest that comorbidities [38, 40] and subclinical autoimmune phenomena [60] might predispose for VAM.
So far available, histological studies heterogeneously describe SARS-CoV‑2 VAM with neutrophilic infiltrates [61], not further specified mixed infiltrates [62], or dominant lymphoplasmatic infiltrates [15]. This contrasts with the clinical expectation of eosinophilic hypersensitivity myocarditis [41], as it was observed in conjunction with other vaccinations [63]. This again raises the questions whether the reported cases are “real” VAMs or coincident findings. Regarding the millionfold administration of mRNA vaccines it seems likely that coincidences of vaccination and a non-VAM occur.
Due to the need for valid and comparable data on mRNA-VAM the diagnostic process should be standardized. So, a somehow “underlying” diagnostic algorithm as a common basis is necessary. Additionally, (more likely) differential diagnoses (e.g. alcoholic cardiomyopathy [64]), local (e.g. regionally varying viruses associated with myocarditis [37]) and case-specific circumstances (e.g. known history of clozapine intake, as potential hint for drug-induced myocarditis [65]) must be considered. So, local experts should be included in the work-up to address “typical local” differential diagnoses in the postmortem setting to ensure valid data on mRNA-VAM. For a sufficient diagnosis of VAM factors potentially influencing the diagnostic process such as the postmortem interval should be considered (see exclusion criteria Fig. 1).
As a derivative from the presented review Fig. 1 suggests a basic diagnostic algorithm for mRNA-VAM. How such an algorithm is applied, depends on the institution. That means, what is sufficient, and efficient should be answered individually for and in each institution and case.
Analogous to the clinical data on time of onset of suspected VAM after vaccination (see supplement), cases with a time of onset of up to 6 weeks post-vaccination should be included in the work-up. Thereby, it seems that people status post SARS-CoV-2-infection present with onset within hours [66]. For this case, infiltrates with Cluster of differentiation (CD) 68-positive macrophages and CD3-positive T‑cells are described [66]. Especially in these cases a differential work-up is mandatory to sufficiently determine whether the myocarditis is viral (i.e. induced due to SARS-CoV-2) or likely caused by the vaccine [66]. The work-up of such cases allows both better data on mRNA vaccine complications and better understanding of the pathophysiological processes in COVID-19 [66].
Limitations
The mRNA SARS-CoV‑2 VAM is a recent topic with rapidly increasing numbers of articles on this issue. So, the presented study tried to include as many articles as possible by means of a basic literature research algorithm, but recurring searches and thus reviews will be necessary for a sustainable knowledge transfer providing guidance for future research. This review focused on the mRNA-VAM.
Conclusion
Valid diagnosis of messenger ribonucleic acid (mRNA)-vaccination associated myocarditis (VAM) is a diagnosis of exclusion requiring consideration of local and case-specific circumstances. Thereby, a high level of pathological expertise, complemented by an extensive differential and interdisciplinary work-up is required. Autopsy series of cases with suspected mRNA severe acute respiratory syndrome-corona virus‑2 (SARS-CoV‑2) VAM are the basis for valid data and deeper pathophysiological understanding. Therefore, a systematic, differential, and standardized diagnostic approach is necessary.
Conclusions for practice
-
Autopsy series are required to generate epidemiological and pathophysiological data on messenger ribonucleic acid (mRNA) severe acute respiratory syndrome-corona virus‑2 (SARS-CoV‑2) vaccination-associated myocarditis.
-
The mRNA vaccination-associated myocarditis is a diagnosis of exclusion requiring exclusion of more likely causes (such as viral myocarditis) and sufficient histological work-up.
-
In view of a millionfold application coincidences between a mRNA vaccination and a non-vaccination-associated myocarditis appear to be likely to occur.
-
Standardized and interdisciplinary diagnostic algorithms are necessary to reliably detect fatal mRNA SARS-CoV‑2 vaccination-associated myocarditis
References
Cited literature
RECOVERY Collaborative Group, Horby P, Lim WS, Emberson JR et al (2021) Dexamethasone in hospitalized patients with Covid-19. N Engl J Med 384(8):693–704
Viana J, van Dorp CH, Nunes A et al (2021) Controlling the pandemic during the SARS-CoV‑2 vaccination rollout. Nat Commun 12(1):3674
Fisman DN, Tuite RA (2021) Progressive increase in virulence of novel SARS-CoV‑2 variants in Ontario, Canada (medRxiv)
Christensen PA, Olsen RJ, Long SW et al (2022) Delta variants of SARS-CoV‑2 cause significantly increased vaccine breakthrough COVID-19 cases in Houston, Texas. Am J Pathol 192(2):320–331. https://doi.org/10.1016/j.ajpath.2021.10.019
Lopez Bernal J, Andrews N, Gower C et al (2021) Effectiveness of Covid-19 vaccines against the B.1.617.2 (delta) variant. N Engl J Med 385(7):585–594
Chia PY, Ong SWX, Chiew CJ et al (2021) Virological and serological kinetics of SARS-CoV‑2 delta variant vaccine breakthrough infections: a multicentre cohort study. Clin Microbiol Infect. https://doi.org/10.1016/j.cmi.2021.11.010
CDC COVID-19 Vaccine Breakthrough Case Investigations Team (2021) COVID-19 vaccine breakthrough infections reported to CDC—United States, January 1‑April 30, 2021. Mmwr Morb Mortal Wkly Rep 70(21):792–793
Chakraborty C, Sharma AR, Bhattacharya M et al (2021) From COVID-19 to cancer mRNA vaccines: moving from bench to clinic in the vaccine landscape. Front Immunol 12:679344
Pardi N, Hogan MJ, Porter FW et al (2018) mRNA vaccines—a new era in vaccinology. Nat Rev Drug Discov 17(4):261–279
Amanna I, Slifka MK (2005) Public fear of vaccination: separating fact from fiction. Viral Immunol 18(2):307–315
Kaplan RM, Milstein A (2021) Influence of a COVID-19 vaccine’s effectiveness and safety profile on vaccination acceptance. Proc Natl Acad Sci U S A 118(10):e2021726118
Dionne A, Sperotto F, Chamberlain S et al (2021) Association of myocarditis with BNT162b2 messenger RNA COVID-19 vaccine in a case series of children. JAMA Cardiol 6(12):1446–1450
Leone O, Pieroni M, Rapezzi C et al (2019) The spectrum of myocarditis: from pathology to the clinics. Virchows Arch 475(3):279–301
Tschöpe C, Ammirati E, Bozkurt B et al (2021) Myocarditis and inflammatory cardiomyopathy: current evidence and future directions. Nat Rev Cardiol 18(3):169–193
Schneider J, Sottmann L, Greinacher A et al (2021) Postmortem investigation of fatalities following vaccination with COVID-19 vaccines. Int J Legal Med 135(6):2335–2345
Suzuki Y, Ishihara H (2021) Difference in the lipid nanoparticle technology employed in three approved siRNA (Patisiran) and mRNA (COVID-19 vaccine) drugs. Drug Metab Pharmacokinet 41:100424
Tano E, San Martin S, Girgis S et al (2021) Perimyocarditis in adolescents after Pfizer-BioNTech COVID-19 vaccine. J Pediatric Infect Dis Soc 10(10):962–966
Deb A, Abdelmalek J, Iwuji K et al (2021) Acute myocardial injury following COVID-19 vaccination: a case report and review of current evidence from vaccine adverse events reporting system database. J Prim Care Community Health 12:21501327211029230
Bautista García J, Peña Ortega P, Bonilla Fernández JA et al (2021) Miocarditis aguda tras administración de vacuna BNT162b2 contra la COVID-19. Rev Esp Cardiol 74(9):812–814
Kim HW, Jenista ER, Wendell DC et al (2021) Patients with acute myocarditis following mRNA COVID-19 vaccination. JAMA Cardiol 6(10):1196–1201
Muthukumar A, Narasimhan M, Li QZ et al (2021) In-depth evaluation of a case of presumed myocarditis after the second dose of COVID-19 mRNA vaccine. Circulation 144(6):487–498
Shaw KE, Cavalcante JL, Han BK et al (2021) Possible association between COVID-19 vaccine and myocarditis: clinical and CMR findings. JACC Cardiovasc Imaging 14(9):1856–1861. https://doi.org/10.1016/j.jcmg.2021.06.002
Chamling B, Vehof V, Drakos S et al (2021) Occurrence of acute infarct-like myocarditis following COVID-19 vaccination: just an accidental co-incidence or rather vaccination-associated autoimmune myocarditis? Clin Res Cardiol 110(11):1850–1854
Sokolska JM, Kurcz J, Kosmala W (2021) Every rose has its thorns—acute myocarditis following COVID-19 vaccination. Kardiol Pol 79(10):1153–1154. https://doi.org/10.33963/KP.a2021.0075
Rosner CM, Genovese L, Tehrani BN et al (2021) Myocarditis temporally associated with COVID-19 vaccination. Circulation 144(6):502–505
Larson KF, Ammirati E, Adler ED et al (2021) Myocarditis after BNT162b2 and mRNA-1273 vaccination. Circulation 144(6):506–508
Abu Mouch S, Roguin A, Hellou E et al (2021) Myocarditis following COVID-19 mRNA vaccination. Vaccine 39(29):3790–3793
Park J, Brekke DR, Bratincsak A (2022) Self-limited myocarditis presenting with chest pain and ST segment elevation in adolescents after vaccination with the BNT162b2 mRNA vaccine. Cardiol Young 32(1):146–149. https://doi.org/10.1017/S1047951121002547
Watkins K, Griffin G, Septaric K et al (2021) Myocarditis after BNT162b2 vaccination in a healthy male. Am J Emerg Med 50:815.e1–815.e2
Mansour J, Short RG, Bhalla S et al (2021) Acute myocarditis after a second dose of the mRNA COVID-19 vaccine: a report of two cases. Clin Imaging 78:247–249
Isaak A, Feisst A, Luetkens JA (2021) Myocarditis following COVID-19 vaccination. Radiology. https://doi.org/10.1148/radiol.2021211766
Hudson B, Mantooth R, DeLaney M (2021) Myocarditis and pericarditis after vaccination for COVID-19. J Am Coll Emerg Physicians Open 2(4):e12498
Marshall M, Ferguson ID, Lewis P et al (2021) Symptomatic acute myocarditis in seven adolescents following Pfizer-BioNtech COVID-19 vaccination. Pediatrics 148(3):e2021052478. https://doi.org/10.1542/peds.2021-052478
Williams CB, Choi JI, Hosseini F et al (2021) Acute myocarditis following mRNA-1273 SARS-coV‑2 vaccination. CJC Open 3(11):1410–1412. https://doi.org/10.1016/j.cjco.2021.07.008
Cereda A, Conca C, Barbieri L et al (2021) Acute myocarditis after the second dose of SARS-CoV‑2 vaccine: serendipity or atypical causal relationship? Anatol J Cardiol 25(7):522–523
D’Angelo T, Cattafi A, Carerj ML et al (2021) Myocarditis after SARS-CoV‑2 vaccination: a vaccine-induced reaction? Can J Cardiol 37(10):1665–1667. https://doi.org/10.1016/j.cjca.2021.05.010
Golpour A, Patriki D, Hanson PJ et al (2021) Epidemiological impact of myocarditis. J Clin Med 10(4):603
Heymans S, Cooper LT (2022) Myocarditis after COVID-19 mRNA vaccination: clinical observations and potential mechanisms. Nat Rev Cardiol 19(2):75–77. https://doi.org/10.1038/s41569-021-00662-w
Dal Ferro M, Bussani R, Paldino A et al (2021) SARS-CoV‑2, myocardial injury and inflammation: insights from a large clinical and autopsy study. Clin Res Cardiol 110(11):1822–1831
Witberg G, Barda N, Hoss S et al (2021) Myocarditis after Covid-19 vaccination in a large health care organization. N Engl J Med 385(23):2132–2139
Montgomery J, Ryan M, Engler R et al (2021) Myocarditis following immunization with mRNA COVID-19 vaccines in members of the US military. JAMA Cardiol 6(10):1202–1206. https://doi.org/10.1001/jamacardio.2021.2833
Satoh H, Sano M, Suwa K et al (2014) Distribution of late gadolinium enhancement in various types of cardiomyopathies: significance in differential diagnosis, clinical features and prognosis. World J Cardiol 6(7):585–601
Chow LH, Radio SJ, Sears TD et al (1989) Insensitivity of right ventricular endomyocardial biopsy in the diagnosis of myocarditis. J Am Coll Cardiol 14(4):915–920
Dettmeyer RB (2011) Forensic histopathology—fundamentals and perspectives, 1st edn. Springer, Berlin, Heidelberg, p 251
Chen W, Jeudy J (2019) Assessment of myocarditis: cardiac MR, PET/CT, or PET/MR? Curr Cardiol Rep 21(8):76
Caredda G (2022) Editorial comment: cardiac MRI as a fundamental tool in the evaluation of suspected myocarditis after COVID-19 mRNA vaccination in young patients. AJR Am J Roentgenol 218:658–658
Maier A, Braig M, Jakob K et al (2020) Molecular magnetic resonance imaging of activated platelets allows noninvasive detection of early myocarditis in mice. Sci Rep 10(1):13211
Guidi B, Aquaro GD, Gesi M et al (2018) Postmortem cardiac magnetic resonance in sudden cardiac death. Heart Fail Rev 23(5):651–665
Shanes JG, Ghali J, Billingham ME et al (1987) Interobserver variability in the pathologic interpretation of endomyocardial biopsy results. Circulation 75(2):401–405
Baughman KL (2006) Diagnosis of myocarditis: death of Dallas criteria. Circulation 113(4):593–595
Hauck AJ, Kearney DL, Edwards WD (1989) Evaluation of postmortem endomyocardial biopsy specimens from 38 patients with lymphocytic myocarditis: implications for role of sampling error. Mayo Clin Proc 64(10):1235–1245
Grabellus F, Mall G, Schnabel PA et al (2005) Immunohistochemical differentiation of eosinophilic heart diseases using antibodies against eosinophil activation markers. Histopathology 46(1):89–97
Basso C, Calabrese F, Angelini A et al (2013) Classification and histological, immunohistochemical, and molecular diagnosis of inflammatory myocardial disease. Heart Fail Rev 18(6):673–681
Fung G, Luo H, Qiu Y et al (2016) Myocarditis. Circ Res 118(3):496–514
Yuan Z, Shioji K, Kishimoto C (2003) Immunohistological analyses of myocardial infiltrating cells in various animal models of myocarditis. Exp Clin Cardiol 8(1):13–16
Klingel K, Sauter M, Bock CT et al (2004) Molecular pathology of inflammatory cardiomyopathy. Med Microbiol Immunol 193(2–3):101–107
Maisch B (2016) Alcoholic cardiomyopathy: the result of dosage and individual predisposition. Herz 41(6):484–493
Olbrich HG (2001) Epidemiology-etiology of dilated cardiomyopathy. Z Kardiol 90:2–9
Elhassan M, Ahmad H, Mohamed M et al (2021) From muscles to wires: report of two cases and literature review on COVID-19 vaccination and cardiac conduction disturbance. Cureus 13(10):e18805
Badshah M, Shriver J, Rynders B et al (2021) MODERNA mRNA-1273 vaccine-associated myopericarditis in a patient with a subclinical autoimmune predisposition. J Cardiol Cases 24(5):227–229
Choi S, Lee S, Seo JW et al (2021) Myocarditis-induced sudden death after BNT162b2 mRNA COVID-19 vaccination in Korea: case report focusing on histopathological findings. J Korean Med Sci 36(40):e286
Verma AK, Lavine KJ, Lin CY (2021) Myocarditis after Covid-19 mRNA vaccination. N Engl J Med 385(14):1332–1334
Engler RJM, Nelson MR, Collins LC Jr et al (2015) A prospective study of the incidence of myocarditis/pericarditis and new onset cardiac symptoms following smallpox and influenza vaccination. PLoS ONE 10(3):e118283
Dettmeyer R, Reith K, Madea B (2002) Alcoholic cardiomyopathy versus chronic myocarditis-immunohistological investigations with LCA, CD3, CD68 and tenascin. Forensic Sci Int 126(1):57–62
Haas SJ, Hill R, Krum H et al (2007) Clozapine-associated myocarditis: a review of 116 cases of suspected myocarditis associated with the use of clozapine in Australia during 1993–2003. Drug Saf 30(1):47–57
Nguyen TD, Mall G, Westphal JG et al (2021) Acute myocarditis after COVID-19 vaccination with mRNA-1273 in a patient with former SARS-CoV‑2 infection. ESC Heart Fail 8(6):4710–4714
Further reading
McLean K, Johnson TJ (2021) Myopericarditis in a previously healthy adolescent male following COVID-19 vaccination: a case report. Acad Emerg Med 28(8):918–921. https://doi.org/10.1111/acem.14322
Singh B, Kaur P, Cedeno L et al (2021) COVID-19 mRNA vaccine and myocarditis. Eur J Case Rep Intern Med 8(7):2681
Vidula MK, Ambrose M, Glassberg H et al (2021) Myocarditis and other cardiovascular complications of the mRNA-based COVID-19 vaccines. Cureus 13(6):e15576
Albert E, Aurigemma G, Saucedo J et al (2021) Myocarditis following COVID-19 vaccination. Radiol Case Rep 16(8):2142–2145
Habib MB, Hamamyh T, Elyas A et al (2021) Acute myocarditis following administration of BNT162b2 vaccine. IDCases 25:e1197
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
J. M. Federspiel, F. Ramsthaler, M. Kettner, and G. Mall declare that they have no competing interests.
For this article no studies with human participants or animals were performed by any of the authors.
Additional information
Jan M. Federspiel and Frank Ramsthaler contributed equally to the manuscript.

Scan QR code & read article online
Supplementary Information
Rights and permissions
About this article
Cite this article
Federspiel, J.M., Ramsthaler, F., Kettner, M. et al. Diagnostics of messenger ribonucleic acid (mRNA) severe acute respiratory syndrome-corona virus‑2 (SARS-CoV‑2) vaccination-associated myocarditis—A systematic review. Rechtsmedizin 33, 125–131 (2023). https://doi.org/10.1007/s00194-022-00587-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00194-022-00587-9
Keywords
- Interdisciplinary work-up
- mRNA-vaccination associated death
- Autopsy
- Diagnostic algorithm
- Differential diagnosis