Abstract
Introduction and hypothesis
Suspension of midurethral sling (MUS) surgery in the UK has led to a call for further evidence regarding long-term morbidity and the efficacy of treatments when mesh complications are encountered. We reviewed how many patients who underwent MUS surgery in Teesside, UK, returned to theatre due to a complication and what the outcomes were following this surgical intervention.
Methods
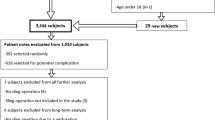
All patients coded to have undergone an MUS procedure between 1 January 2010 and 31 December 2014 in Teesside were reviewed retrospectively (n = 924). Case notes were analysed for patients who returned to theatre up until December 2017 due to complications related to their original MUS.
Results
Seventy-one of 924 (7.7%) women returned to theatre for some form of surgical intervention. There was a statistically significant difference in return-to-theatre rate between the transobturator and retropubic approach groups (63/661; 9.5%; confidence interval (CI) 7.3–11.8% v 8/263; 3.0%; CI 0.96%, 5.1%, odds ratio (OR) 3.35, p = 0.001); 2.8% (26/924) underwent shortening, reburying, incision or MUS excision; 1.0% (9/924) underwent steroid injection along the MUS tract; 1.7% (16/924) underwent surgical treatment of detrusor overactivity; 3.0% (28/924) required further stress incontinence surgery. The risk of unresolved chronic pain post-MUS surgery following treatment of complications was 0.2% (2/924).
Conclusions
Our results show a reassuringly low rate of mesh removal following MUS surgery. Furthermore, outcomes were good following surgical management of MUS complications. We advocate compulsory registration of all MUS procedures, follow-up data and complications to provide robust long-term evidence for the future.
Similar content being viewed by others
References
“Scandal” of vaginal mesh removal rates revealed by NHS records. Available from: https://www.theguardian.com/society/2017/aug/15/scandal-of-vaginal-mesh-removal-rates-revealed-by-nhs-records. Accessed 10 Oct 2018.
More than 800 women sue NHS and manufacturers over vaginal mesh implants. Available from: https://www.theguardian.com/society/2017/apr/18/more-than-800-women-sue-nhs-and-manufacturers-over-vaginal-mesh-implants. Accessed 10 Oct 2018.
Mesh Working Group Interim Report. Available from: https://www.england.nhs.uk/wp-content/uploads/2015/12/mesh-wg-interim-rep.pdf. Accessed 10 Oct 2018.
Varley D (2012) Summaries of the safety/adverse effects of vaginal tapes/slings/meshes for stress urinary incontinence and prolapse. 1–72. Available from: http://www.mhra.gov.uk/home/groups/commsic/documents/websiteresources/con205383.pdf. Accessed 10 Oct 2018.
Keltie K, Elneil S, Monga A, et al. Complications following vaginal mesh procedures for stress urinary incontinence: an 8 year study of 92,246 women. Sci Rep. 2017;7:12015. https://doi.org/10.1038/s41598-017-11821-w.
Ford AA, Rogerson L, Cody JD, et al. Mid-urethral sling operations for stress urinary incontinence in women. Cochrane Database Syst Rev. 2017;7:CD006375. https://doi.org/10.1002/14651858.CD006375.pub4.
A summary of the evidence on the benefits and risks of vaginal mesh implants. Available from: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/402162/Summary_of_the_evidence_on_the_benefits_and_risks_of_vaginal_mesh_implants.pdf. Accessed 10 Oct 2018.
Mesh Oversight Group Report. Available from: https://www.england.nhs.uk/wp-content/uploads/2017/07/mesh-oversight-group-report.pdf. Accessed 10 Oct 2018.
Opinion on The safety of surgical meshes used in urogynecological surgery. Available from: https://ec.europa.eu/health/scientific_committees/emerging/docs/scenihr_o_049.pdf. Accessed 10 Oct 2018.
Morling JR, McAllister DA, Agur W, et al. Adverse events after first, single, mesh and non-mesh surgical procedures for stress urinary incontinence and pelvic organ prolapse in Scotland, 1997-2016: a population-based cohort study. Lancet. 2017;389:629–40. https://doi.org/10.1016/S0140-6736(16)32572-7.
Women harmed because vaginal mesh regulation “not fit for purpose.” Available from: https://www.theguardian.com/society/2017/dec/06/woman-great-harm-due-loopholes-vaginal-mesh-regulation. Accessed 10 Oct 2018.
Parliamentary debate- surgical mesh implants. Available from: http://hansard.parliament.uk/commons/2017-10-18/debates/B546B1F1-099F-442C-AD71-0185D1B3F69C/SurgicalMeshImplants. Accessed 10 Oct 2018.
NHS Improvement Provider bulletin 25 July 2018. Available from: https://improvement.nhs.uk/news-alerts/provider-bulletin-25-july-2018/. Accessed 10 Oct 2018.
Recommendations of the Mesh Pause Clinical Advisory Group to Medical Directors and Surgical Teams. Available from: https://www.rcog.org.uk/globalassets/documents/guidelines/safety-alerts/meshclinicaladvisorygroupcmoadvice.pdf. Accessed 10 Oct 2018.
Glazener CM, Breeman S, Elders A, et al. Mesh, graft, or standard repair for women having primary transvaginal anterior or posterior compartment prolapse surgery: two parallel-group, multicentre, randomised, controlled trials (PROSPECT). Lancet. 2017;389:381–92. https://doi.org/10.1016/S0140-6736(16)31596-3.
Jha S, Cutner A, Moran P. The UK national prolapse survey: 10 years on. Int Urogynecol J. 2018;29:795–801. https://doi.org/10.1007/s00192-017-3476-3.
BSUG- Definition of a Urogynaecologist. Available from: http://www.bsug.org.uk/pages/about/definition-of-urogynaecologist/84. Accessed 10 Oct 2018.
Long C-Y, Lo T-S, Liu C-M, et al. Lateral excision of tension-free vaginal tape for the treatment of iatrogenic urethral obstruction. Obstet Gynecol. 2004;104:1270–4. https://doi.org/10.1097/01.AOG.0000146282.51404.93.
Roth TM. Management of persistent groin pain after transobturator slings. Int Urogynecol J Pelvic Floor Dysfunct. 2007;18:1371–3. https://doi.org/10.1007/s00192-007-0365-1.
Duckett JRA, Jain S. Groin pain after a tension-free vaginal tape or similar suburethral sling: management strategies. BJU Int. 2005;95:95–7. https://doi.org/10.1111/j.1464-410X.2004.05258.x.
Management of vaginal mesh erosion following the tension free vaginal; tape procedure: five years experience at a UK tertiary referral centre. Available from: https://www.ics.org/Abstracts/Publish/105/000763.pdf. Accessed 10 Oct 2018.
Welk B, Al-Hothi H, Winick-Ng J. Removal or revision of vaginal mesh used for the treatment of stress urinary incontinence. JAMA Surg. 2015;150:1167–75. https://doi.org/10.1001/jamasurg.2015.2590.
Lanier ST, Wang ED, Chen JJ, et al. The effect of acellular dermal matrix use on complication rates in tissue expander/implant breast reconstruction. Ann Plast Surg. 2010;64:674–8. https://doi.org/10.1097/SAP.0b013e3181dba892.
Matharu GS, Pynsent PB, Dunlop DJ. Revision of metal-on-metal hip replacements and resurfacings for adverse reaction to metal debris: a systematic review of outcomes. Hip Int. 2014;24:311–20. https://doi.org/10.5301/hipint.5000140.
Albino FP, Patel KM, Nahabedian MY, et al. Does mesh location matter in abdominal wall reconstruction? A systematic review of the literature and a summary of recommendations. Plast Reconstr Surg. 2013;132:1295–304. https://doi.org/10.1097/PRS.0b013e3182a4c393.
Noblett K, Benson K, Kreder K. Detailed analysis of adverse events and surgical interventions in a large prospective trial of sacral neuromodulation therapy for overactive bladder patients. Neurourol Urodyn. 2017;36:1136–9. https://doi.org/10.1002/nau.23076.
Balzarro M, Rubilotta E, Goss C, et al. Counseling in urogynecology: a difficult task, or simply good surgeon-patient communication? Int Urogynecol J. 2018;29:943–8. https://doi.org/10.1007/s00192-018-3673-8.
NICE Guideline 171: Urinary Incontinence in Women. Available from: https://www.nice.org.uk/guidance/cg171. Accessed 10 Oct 2018.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
V Kershaw: None.
R Nicholson: None.
P Ballard: None.
A Khunda: Received an educational travel grant from Medtronic plc.
S Puthuraya: None.
E Gouk: None.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Conference Presentations
International Urogynecological Association Anuual Meeting, Vienna, 27–30 June 2018; United Kingdom Continence Society Annual Scientific Meeting, Telford, 18–20 April 2018
Rights and permissions
About this article
Cite this article
Kershaw, V., Nicholson, R., Ballard, P. et al. Outcome of surgical management for midurethral sling complications: a multicentre retrospective cohort study. Int Urogynecol J 31, 329–336 (2020). https://doi.org/10.1007/s00192-018-3853-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-018-3853-6