Abstract
Introduction and hypothesis
Class action against Ethicon (J&J), manufacturer of transvaginal mesh devices, including mid-urethral slings (MUS), was brought to the Federal Court of Australia in 2016 by Shine Lawyers. As a result, subpoenas to all hospitals and networks were received, which overrode patient privacy concerns. This medical record search allowed a complete audit and communication with patients to offer clinical review. This enabled a review of complications, readmission and re-operation for women who underwent a MUS for stress urinary incontinence.
Methods
A cohort study of women who underwent MUS treatment for stress urinary incontinence (SUI) at a single tertiary teaching hospital between 1999 and 2017 was carried out. The main outcome measures were the rate of readmission and re-operation following MUS procedures. These include voiding dysfunction managed by sling loosening or sling division, mesh pain or exposure managed by mesh removal and reoperation for recurrent stress urinary incontinence.
Results
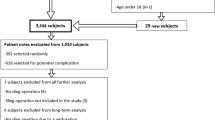
Between 1999 and 2017, a total of 1,462 women were identified as having a MUS; of these, 1,195 (81.7%) had full patient records available. Voiding dysfunction requiring surgical intervention with sling loosening or division was 3%, excision for mesh exposure was 2%, and partial or complete excision for pain was 1% at a median of 10 years from index surgery. The reoperation rate for recurrent stress urinary incontinence was 3%.
Conclusion(s)
This audit of all MUS procedures performed at a tertiary centre confirms an overall low rate of readmission for complications and recurrent SUI surgery; this justifies its continued availability with appropriate informed consent.

Similar content being viewed by others
References
Luber KM. The definition, prevalence, and risk factors for stress urinary incontinence. Rev Urol. 2004;6(Suppl 3):S3–9.
Margalith I, Gillon G, Gordon D. Urinary incontinence in women under 65: quality of life, stress related to incontinence and patterns of seeking health care. Qual Life Res. 2004;13(8):1381–90. https://doi.org/10.1023/B:QURE.0000040794.77438.cf.
Lee J, Dwyer PL. Age-related trends in female stress urinary incontinence surgery in Australia—Medicare data for 1994–2009. Aust N Z J Obstet Gynaecol. 2010;50(6):543–9. https://doi.org/10.1111/j.1479-828X.2010.01217.x.
Ford AA, Rogerson L, Cody JD, Aluko P, Ogah JA. Mid-urethral sling operations for stress urinary incontinence in women. Cochrane Database Syst Rev. 2017;7(7):CD006375. https://doi.org/10.1002/14651858.CD006375.pub4.
Fusco F, Abdel-Fattah M, Chapple CR, et al. Updated systematic review and meta-analysis of the comparative data on colposuspensions, pubovaginal slings, and midurethral tapes in the surgical treatment of female stress urinary incontinence. Eur Urol. 2017;72(4):567–91. https://doi.org/10.1016/j.eururo.2017.04.026.
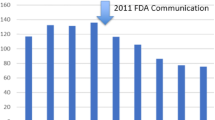
FDA Public Health Notification: Serious complications associated with transvaginal placement of surgical mesh in repair of pelvic organ prolapse and stress urinary incontinence. http://wayback.archiveit.org/7993/20170111190506/http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/PublicHealthNotifications/ucm061976.htm.
Souders CP, Eilber KS, McClelland L, et al. The truth behind transvaginal mesh litigation: devices, timelines, and provider characteristics. Female Pelvic Med Reconstr Surg. 2018;24(1):21–5. https://doi.org/10.1097/SPV.0000000000000433.
Dyer O. Johnson and Johnson faces lawsuit over vaginal mesh devices. BMJ. 2016;353:i3045. https://doi.org/10.1136/bmj.i3045.
Dolan L. The controversy of polypropylene mesh. Obstet Gynaecol Reprod Med. 2018;28(10):329–31.
Thompson C, Faunce T. Australian Senate Committee report on transvaginal mesh devices. J Law Med. 2018;25(4):934–43.
Australian Commission on Safety and Quality in Health Care. Treatment options for complications of transvaginal mesh (including options for mesh removal). https://www.safetyandquality.gov.au/publications-and-resources/resource-library/treatment-options-complications-transvaginal-mesh-including-options-mesh-removal.
Cundiff GW, Quinlan DJ, van Rensburg JA, Slack M. Foundation for an evidence-informed algorithm for treating pelvic floor mesh complications: a review. BJOG. 2018;125(8):1026–37. https://doi.org/10.1111/1471-0528.15148.
Mathieson R, Kippen R, Manning T, Brennan J. Stress urinary incontinence in the mesh complication era: current Australian trends. BJU Int. 2021;128(1):95–102. https://doi.org/10.1111/bju.15302.
Norton PA, Nager CW, Chai TC, et al. Risk factors for incomplete bladder emptying after midurethral sling. Urology. 2013;82(5):1038–41. https://doi.org/10.1016/j.urology.2013.05.060.
Chung SM, Moon YJ, Jeon MJ, Kim SK, Bai SW. Risk factors associated with voiding dysfunction after anti-incontinence surgery. Int Urogynecol J. 2010;21(12):1505–9. https://doi.org/10.1007/s00192-010-1229-7.
Rardin CR, Rosenblatt PL, Kohli N, Miklos JR, Heit M, Lucente VR. Release of tension-free vaginal tape for the treatment of refractory postoperative voiding dysfunction. Obstet Gynecol. 2002;100(5 Pt 1):898–902. https://doi.org/10.1016/s0029-7844(02)02279-2.
Albo ME, Richter HE, Brubaker L, et al. Burch colposuspension versus fascial sling to reduce urinary stress incontinence. N Engl J Med. 2007;356(21):2143–55. https://doi.org/10.1056/NEJMoa070416.
Gurol-Urganci I, Geary RS, Mamza JB, et al. Long-term rate of mesh sling removal following midurethral mesh sling insertion among women with stress urinary incontinence. JAMA. 2018;320(16):1659–69. https://doi.org/10.1001/jama.2018.14997.
Hilton P, Rose K. The "learning curve" for retropubic mid-urethral sling procedures: a retrospective cohort study. Int Urogynecol J. 2016;27(4):565–70. https://doi.org/10.1007/s00192-015-2853-z.
Dyer C. Johnson & Johnson loses class action over vaginal mesh in Australia. BMJ. 2019;367:l6659. https://doi.org/10.1136/bmj.l6659.
Therapeutic Goods Administration (TGA). 2022. Information for medical practitioners on up-classification of surgical mesh devices. Available at: Accessed 23 Feb 2022.
Acknowledgements
The authors would like to thank Prof. Beverley Vollenhoven (Head of Gynaecology, Monash Health) Peter Ryan (Chief Legal Officer, Monash Health), Emma Pelham-Thorman (legal department, Monash Health) and Hayley Capiron (Medical Information Technology) for their involvement in the review.
Authors’ contributions
M. Kulkarni: study design, database, manuscript preparation; Y. Liu: database, manuscript preparation; M. Silagy: database, manuscript preparation; D. Rolnik: statistical analysis, manuscript preparation; A. Rosamilia: study design, database, manuscript preparation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 14 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kulkarni, M., Liu, Y., Silagy, M. et al. The transvaginal mesh class action: a tertiary teaching hospital experience of all mid-urethral sling procedures performed between 1999 and 2017. Int Urogynecol J 34, 2573–2580 (2023). https://doi.org/10.1007/s00192-023-05575-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-023-05575-5