Abstract
Introduction
This narrative review summarizes current knowledge on the physiology and pathophysiology of expiratory muscle function in ICU patients, as shared by academic professionals from multidisciplinary, multinational backgrounds, who include clinicians, clinical physiologists and basic physiologists.
Results
The expiratory muscles, which include the abdominal wall muscles and some of the rib cage muscles, are an important component of the respiratory muscle pump and are recruited in the presence of high respiratory load or low inspiratory muscle capacity. Recruitment of the expiratory muscles may have beneficial effects, including reduction in end-expiratory lung volume, reduction in transpulmonary pressure and increased inspiratory muscle capacity. However, severe weakness of the expiratory muscles may develop in ICU patients and is associated with worse outcomes, including difficult ventilator weaning and impaired airway clearance. Several techniques are available to assess expiratory muscle function in the critically ill patient, including gastric pressure and ultrasound.
Conclusion
The expiratory muscles are the "neglected component" of the respiratory muscle pump. Expiratory muscles are frequently recruited in critically ill ventilated patients, but a fundamental understanding of expiratory muscle function is still lacking in these patients.
Similar content being viewed by others
The expiratory muscles are the “neglected component” of the respiratory muscle pump. This narrative review summarizes the physiology and pathophysiology of expiratory muscles in critically ill ventilated patients. Techniques to monitor expiratory muscle function in these patients are also discussed. |
Introduction
The respiratory muscle pump drives alveolar ventilation and is therefore of vital importance. The diaphragm, rib cage muscles and abdominal wall muscles are the most important components of the respiratory muscle pump [1]. Recruitment of each muscle depends on the (relative) load imposed on the respiratory system, lung volume, and the phase of the respiratory cycle. An acute imbalance between respiratory muscle load and capacity will result in respiratory failure and, ultimately, the need for mechanical ventilation. Many studies and reviews have focused on diaphragm structure and function in patients with acute respiratory failure, including critically ill patients [2,3,4,5,6,7,8,9,10,11]. However, the role of expiratory muscles in the physiology of breathing in acute respiratory failure is largely neglected in the literature. This is surprising, given the important role of these muscles in respiration, especially in patients with impending respiratory failure.
The aim of the current paper is to discuss the role of the expiratory muscles in respiration, in particular in critically ill patients in whom respiratory muscle weakness develops rapidly, and may thus have a large clinical impact. We will also describe techniques used to evaluate expiratory muscle function in intensive care unit (ICU) patients. We will not focus in detail on the role of the expiratory muscles in coughing or maintaining body position.
Physiology of expiratory muscle recruitment
The expiratory muscles include those of the abdominal wall (transversus abdominis muscle, internal oblique muscle, external oblique muscle, and rectus abdominis muscle) and some of the rib cage ones (e.g., the internal intercostal muscles and the triangularis sterni muscle) [1, 12,13,14,15,16] (Fig. 1). During tidal breathing, the expiratory muscles are largely inactive, although the transversus abdominis muscle may occasionally show some activity during quiet breathing [16]. Also, in the upright position, the abdominal wall muscles exhibit tonic activity to counteract the gravitational forces acting on the abdominal contents and thus to maintain the diaphragm at optimal length for pressure generation [17,18,19].
The expiratory muscles of the respiratory muscle pump. The respiratory muscle pump is a complex organ that involves a large number of muscles that contribute to inspiration or expiration. This figure schematically demonstrates the expiratory muscles. With the exception of the diaphragm, other inspiratory muscles are not shown
Figure 2 shows the physiology of expiratory muscle recruitment. Activation of the expiratory muscles during breathing occurs when the (relative) load imposed on the inspiratory muscles increases. High absolute respiratory loading may occur under different conditions, such as exercise, low respiratory system compliance, and intrinsic positive end-expiratory pressure (PEEPi). Low inspiratory muscle capacity (high relative load on inspiratory muscles) is common in ICU patients due to ICU-acquired respiratory muscle weakness [20]. In the presence of an imbalance between inspiratory muscle load and capacity, the abdominal wall muscles are recruited during expiration in a fixed hierarchy [21,22,23,24]: initially the transversus abdominis muscle, followed by the internal oblique muscle and the external oblique muscle, and finally the rectus abdominis muscle [16, 17, 25]. Activation of the abdominal wall muscles increases abdominal pressure in the expiratory phase. As the diaphragm is relaxed during (most of the) expiratory phase, this increased abdominal pressure is transmitted to the pleural space, consequently reducing the expiratory transpulmonary pressure, which helps to deflate the lung (less pulmonary hyperinflation/lung strain). Furthermore, increased abdominal pressure enhances inspiratory muscle capacity via at least two mechanisms. First, increased abdominal pressure moves the diaphragm at end expiration to a more cranial position, which results in a more optimal length for tension generation [26, 27]; second, when the end-expiratory lung volume falls below functional residual capacity (FRC), elastic energy is stored in the respiratory system. This stored energy facilitates the next inspiration (i.e., allows more rapid and greater development of negative pleural pressure) [28, 29]. In fact, during strenuous inspiratory loading up to 28% of tidal volume is generated below FRC, which can be attributed to expiratory muscle contraction [21].
Physiology of expiratory muscle recruitment. Schematic illustration of the causes and consequences of expiratory muscle recruitment under physiological (healthy) conditions. All the consequences of expiratory muscle recruitment occur during expiration, except for the increased inspiratory muscle capacity (which occurs during the subsequent inspiration). See main text for explanation. EELV end-expiratory lung volume, PEEPi intrinsic positive end-expiratory pressure, PEEPe external positive end-expiratory pressure
It should be recognized that isolated contraction of the abdominal expiratory muscles causing an increase in abdominal pressure and pleural pressure would result in chest wall distortion, in particular expansion of the lower rib cage. This would likely increase the elastic inspiratory work of breathing and flatten the diaphragm. To limit distortion of the lower rib cage during active expiration, the internal intercostal muscles are recruited to stabilize the rib cage [1].
In addition to an imbalance between inspiratory muscle load and capacity, an increased end-expiratory lung volume, as in application of positive end-expiratory pressure (PEEP), may also recruit the abdominal wall muscles (Figs. 2 and 3) [30]. For example, in patients with normal respiratory system compliance (i.e., 80 mL/cmH2O), application of 10 cmH2O of PEEP would, theoretically, increase end-expiratory lung volume by 800 mL (in the absence of airway closure). However, a physiological feedback mechanism involving vagal pathways or proprioceptive influences limits the increase in end-expiratory lung volume by activation of the abdominal wall muscles during expiration, and thus protects against high lung strain [31, 32].
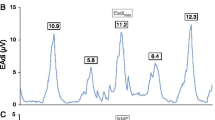
Activation of the abdominal muscles during high PEEP. Tracing of airway pressure (Paw), flow, EMG of the abdominal muscles (EMGabd) and gastric pressure (Pga) obtained from a healthy subject during non-invasive ventilation with PEEP levels of 2 cmH2O (left) and 15 cmH2O (right). At 2 cmH2O of PEEP there is no evidence of activation of the abdominal wall muscles (no EMGabd activity during expiration and no rise in Pga during expiration), however at 15 cmH2O of PEEP, the abdominal muscles are recruited during the expiratory phase, as shown by the presence of EMGabd activity during expiration and the rise in Pga during expiration. White column: inspiration; blue column: expiration. In the Pga tracing obtained during PEEP 15 cmH2O calculation of parameters to estimate expiratory muscle activity are shown: increase in gastric pressure during expiration (ΔPgaexp); and the gastric pressure–time product during expiration (PTPgaexp) represented by the orange area. EMGabd electromyography of abdominal wall muscles, Paw airway pressure, PEEP positive end-expiratory pressure, Pga gastric pressure, PTPgaexp gastric pressure-time product during expiration
Another fundamental role of the expiratory muscles is to develop effective cough pressure to facilitate airway clearance [33]. Contraction of the expiratory muscles against a closed airway may increase the intrathoracic pressure may increase to as high as 300 mmHg within 0.2 s. Once the glottis is open, a very high expiratory flow (up to 720 L/min) can be generated [33, 34]. Expiratory muscle weakness reduces cough strength and peak flow velocity, predisposing patients to pneumonia and atelectasis [33, 35, 36].
Undesirable effects of expiratory muscle recruitment
Recruitment of the expiratory muscles during expiration may have undesirable effects in critically ill patients (Fig. 4 and Table 1).
Pathophysiology of expiratory muscle recruitment. Schematic illustration of the pathophysiological consequences of expiratory muscle recruitment in critically ill patients. The depicted relationships are mostly hypothetical due to the low number of studies on expiratory muscle function in ICU patients. The elevated pleural pressure caused by expiratory muscle recruitment might lead to dynamic airway collapse, especially in patients who already have expiratory flow limitation (EFL). This leads to an equal or increased end-expiratory lung volume (EELV). On the other hand, elevated pleural pressure might lead to negative expiratory transpulmonary pressures, especially in diseases with an increased lung elastance such as in ARDS, which in turn leads to atelectasis and tidal recruitment. EFL expiratory flow limitation, ARDS acute respiratory distress syndrome, VILI ventilator-induced lung injury
First, in patients with acute respiratory distress syndrome (ARDS) or atelectasis, increased pleural pressure during expiration resulting from expiratory muscle recruitment may result in negative transpulmonary pressure during expiration, leading to cyclic alveolar collapse or airway closure and thereby facilitating small airway and alveolar injury [37,38,39,40]. Consistent with this reasoning, a recent study in ARDS patients demonstrated a higher expiratory transpulmonary pressure in patients receiving neuromuscular blockers compared with control patients (1.4 ± 2.7 cmH2O versus − 1.8 ± 3.5 cmH2O, respectively, p = 0.02) [41]. Interestingly, neuromuscular blockers also abolish expiratory activity of the diaphragm (if present) [42] which is expected to decrease expiratory transpulmonary pressure. However, the pressure generated by the diaphragm in the expiratory phase is relatively low compared with that generated by the expiratory muscles. Therefore, the effects of neuromuscular blockers on expiratory transpulmonary pressure largely depend on the relaxation of the expiratory muscles.
Second, expiratory flow limitation is a condition in which expiratory flow cannot be increased, despite an increase in expiratory driving pressure (pressure difference between alveoli and mouth during expiration) [43]. Typically, this occurs in patients with emphysema, but it may also occur during tidal breathing in patients with expiratory muscle activity. The exact mechanism is unclear, but it has been proposed that dynamic airway compression plays an important role [44] (Fig. 5). Elevated pleural pressure during active expiration decreases the airway transluminal pressure, which subsequently may compress the collapsible part of the airway. Total airway collapse is prevented as increased pleural pressure is also transmitted to the alveoli/airways (for an extensive discussion see also [43]). Expiratory airway compression may result in elevated end-expiratory lung volume and PEEPi [43], especially in patients with chronic obstructive pulmonary diseases (COPD) and in patients failing ventilator weaning [24, 45].
Role of expiratory muscle recruitment in the development of expiratory flow limitation (EFL). Schematic and simplified illustration demonstrating the role of expiratory muscle activation in EFL. a–c With activation of the expiratory muscles the abdominal pressure increases, also increasing pleural pressure during expiration. This decreases the transluminal pressure resulting in partial airway collapse and therefore EFL. With higher expiratory muscle pressure the flow-limiting site, or choking point, moves towards the alveoli. Note that gravitational forces are not considered in this illustration. Pab abdominal pressure, Palv alveolar pressure, Pao airway opening pressure, Ppl pleural pressure
Third, in patients weaning from mechanical ventilation, expiratory muscle recruitment is expected when an imbalance exists between the respiratory load and inspiratory muscle capacity. Indeed, activation of the expiratory muscles has been demonstrated during ventilator weaning, especially in patients failing a weaning trial [22,23,24]. We recently found that expiratory muscle effort progressively increased throughout the trial in such patients [24]. The neuromuscular efficiency of the diaphragm was lower in weaning failure patients compared with weaning success patients, which challenges the concept that expiratory muscle activation improves diaphragm contractile efficiency [24], although this requires further evaluation. Nevertheless, recruitment of the expiratory muscles during a weaning trial appears to be a strong marker of weaning failure.
Technically, expiratory muscle activity interferes with the assessment of PEEPi.
PEEPi can be measured using different techniques. In patients with expiratory muscle activity, an end-expiratory occlusion will be highly influenced and exaggerated by the contraction of the expiratory muscles [46]. Similarly, the relaxation of the expiratory muscles at the beginning of the effort explains part of the initial drop in esophageal pressure, which is not entirely explained by so-called dynamic PEEPi. Either the drop in gastric pressure (Pga) or the rise in Pga during expiration must be subtracted from the esophageal drop in order to measure a reliable PEEPi [47].
Expiratory muscle strength in critically ill patients
Several studies have demonstrated the development of expiratory muscle weakness in critically ill patients [48,49,50,51,52,53,54,55,56,57,58,59,60,61,62, 64]. Most studies used the maximum expiratory pressure (MEP) as a marker of expiratory muscle strength [48,49,50,51,52,53,54,55,56,57]. Despite the heterogeneity of the studies in terms of populations and measurement techniques, the MEP was lower than the reference values [63] in all studies that obtained MEP at the time of ventilator weaning [48,49,50,51,52,53,54,55, 64]. Patients failing extubation exhibit a lower MEP (mean decrease varying from 9 to 31 cmH2O) compared with extubation success patients [48,49,50,51,52,53,54,55, 64]. This indicates that expiratory muscle weakness is a potential predictor of weaning outcome. How expiratory muscle weakness affects weaning and extubation outcome is largely unknown. Potential explanations include inadequate secretion clearance and insufficient cough capacity resulting in atelectasis, reduced contractile efficiency of the diaphragm, or inadequate reduction of PEEPi.
Remarkably, no studies have investigated the association between diaphragm weakness and expiratory muscle weakness.
Risk factors for expiratory muscle weakness in critically ill patients
Risk factors for the development of ICU-acquired weakness of the peripheral muscles and diaphragm have been discussed recently [2, 4, 57, 65]. Whether these risk factors also have an impact on the expiratory muscles is largely unknown. We briefly discuss risk factors that may contribute to the development of expiratory muscle weakness.
Sepsis
Sepsis and systematic inflammation have been linked to the development of muscle weakness, including weakness of the expiratory muscles [2, 61, 65]. Sepsis induces a severe and persistent increase in protein catabolism, resulting in muscle wasting and muscle weakness [59, 60]. Compared with non-septic surgical patients, the rectus abdominis muscle from surgical patients with sepsis showed significantly lower in vitro contractility [59]. In addition, the reduced MEP (≤ 30 cmH2O) found at the time patients regained normal consciousness showed an independent association with septic shock [57].
Mechanical ventilation
Mechanical ventilation plays an important role in the development of diaphragmatic dysfunction in critically ill patients [2, 9, 10, 66]. Potential mechanisms include disuse atrophy due to ventilator over-assist, or load-induced injury as a result of ventilator under-assist. The impact of mechanical ventilation on expiratory muscles has not been systematically investigated. However, as mentioned earlier, ventilator settings including PEEP and the level of inspiratory assist may have an impact on the activity of the expiratory muscles (Fig. 3) [46, 67], although the ultimate impact of mechanical ventilation on expiratory muscle strength is largely unknown and should be further investigated.
Other risk factors
Co-morbidities, such as COPD and myopathies, or complications such as intra-abdominal hypertension, may put patients at increased risk of ICU-associated expiratory muscle weakness [68, 69]. Drugs such as sedatives, neuromuscular blockers and corticosteroids have been shown to affect peripheral muscle function and diaphragm muscle function in ICU patients [2, 65, 70]. The effects of these drugs on expiratory muscle function have not been systematically studied.
Strategies to maintain or improve expiratory muscle strength
Quantification of expiratory muscle effort in critically ill patients
While visual inspection of the trunk and palpation of the abdominal wall may reveal activation of the expiratory muscles, they do not allow quantification of effort. In this section, we summarize the main clinical techniques that can be used to quantify expiratory muscle effort in ICU patients.
Gastric pressure
Activation of the abdominal wall muscles increases abdominal pressure. Changes in Pga during expiration reflect changes in abdominal pressure and can thus be used to quantify expiratory muscle effort [22, 24, 39, 63, 73]. Pga is measured using an air-filled balloon catheter inserted into the stomach. Bladder pressure has also been proposed as a means of quantifying intra-abdominal pressure [74, 75], and showed an acceptable correlation with Pga in supine position (bias = 0.5 mmHg, and precision = 3.7 mmHg (limits of agreement, − 6.8 to 7.5 mmHg)) [74]. To quantify the effort of expiratory muscles, Pga amplitude and the Pga pressure–time product (PTP) during expiration can be calculated (Fig. 3).
Amplitude of gastric pressure
Both the rise in Pga over the course of expiration [46] and the drop in Pga at the onset of the next inspiration [76] have been used to quantify the activity of the expiratory muscles. However, only the expiratory increase in Pga showed a good correlation with the electromyographic amplitude of the transverse abdominis muscle (correlation coefficient ranging from 0.70 to 0.95) [77].
Pressure–time product
The PTP of the expiratory muscles has been quantified using the area enclosed by the esophageal pressure curve and the static chest-wall recoil pressure curve during expiration [78]. The PTP accounts for the energy expenditure during both the isometric and dynamic phases of expiration (independently of volume displacement). However, expiratory esophageal pressure only represents the pressure generated by the abdominal wall muscles when the diaphragm is completely relaxed [39, 79]. As diaphragm activity has been demonstrated during expiration [42, 67], abdominal wall muscle effort cannot be reliably quantified using the expiratory esophageal PTP alone. Therefore, it is recommended to use the expiratory Pga in order to calculate the PTP of the expiratory muscles [80,81,82,83]. The gastric PTP can be obtained from the area under the expiratory Pga curve, in which the baseline is defined as the resting end-expiratory Pga from the preceding breath [24, 80, 81].
Work of breathing
Traditionally, the Campbell diagram is used to quantify the inspiratory work of breathing [84], but it allows estimation of the expiratory work as well. The area of the esophageal pressure–volume loop at the right side of the chest wall relaxation curve represents expiratory muscle effort [85, 86]. By definition, work is performed only when there is volume displacement (work = pressure × volume). However, as explained above, during dynamic airway collapse part of the pressure generated by the expiratory muscles does not result in lung volume displacement, and therefore the Campbell diagram underestimates the total effort of the expiratory muscles [44, 87]. Under these circumstances, the PTP may better reflect expiratory muscle effort.
Volitional tests of expiratory muscle strength
The MEP is the most widely used measure of expiratory muscle strength [63]. Standard procedures for non-intubated subjects have been established [63]. For intubated patients, the MEP can be measured using a unidirectional valve that allows inspiration but prevents expiration [48, 51, 88]. Some investigators coached subjects to perform an expiratory effort against an occluded airway for 25 to 30 s, and then recorded the most positive pressure developed [48, 51, 88]. Calculating the ratio of maximum inspiratory pressure to MEP is a simple way to assess the relative impairment of the inspiratory muscles versus the expiratory muscles [89]. As MEP measurement requires a voluntary patient effort, this might not be feasible in a proportion of ICU patients. As an alternative to MEP, cough pressure can be assessed to quantify expiratory muscle strength [33, 63, 73].
Cough test
The cough test is a relatively easy-to-perform, complementary test for the diagnosis of expiratory muscle weakness. Both cough pressure measured via air-filled balloons in the stomach or esophagus, and cough peak expiratory flow measured at the opening of an endotracheal tube or using the ventilator flow sensor [90], are feasible in ICU patients. In patients unable to cooperate, a cough may be induced either by instilling physiological saline [35] or by advancing a suctioning catheter through the patient’s tube [36].
Abdominal wall muscle ultrasound
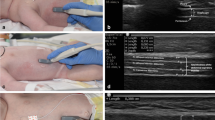
Ultrasound has become a popular tool for quantifying changes in the thickness and activity of the diaphragm in ICU patients [3, 91, 92], but few studies have used this technique to evaluate the expiratory muscles. Abdominal ultrasound allows direct visualization of the three layers of the abdominal wall muscles and the rectus abdominis muscle [93,94,95,96] (Fig. 6). In our experience, the abdominal wall muscles are easy to visualize using ultrasound, and measurement of thickness is feasible in almost all patients. In healthy subjects, the thickness of individual abdominal wall muscles follows a certain pattern: transversus abdominis < external oblique < internal oblique < rectus abdominis [96]. The thickness of the transversus abdominis muscle measured with ultrasound is strongly correlated with the pressure developed during an expiratory maneuver (assessed by the change in Pga) [94]. In addition, the transversus abdominis muscle thickness increase is significantly correlated with the muscle’s electrical activity [93]. However, all these studies were performed in healthy subjects, and further studies are needed to determine the reliability and validity of ultrasound assessment of expiratory muscle thickness and function in ICU patients.
Ultrasound image of the abdominal muscles. Left: ultrasound image of the rectus abdominis muscle (RA) (top), obtained with the probe placed 2–3 cm above the umbilicus and 2–3 cm from the midline (bottom). Right: ultrasound image of the external oblique muscle (EO), internal oblique muscle (IO) and transversus abdominis muscle (TrA) (top), obtained with the probe placed midway between the costal margin and the iliac crest, along the anterior axillary line (bottom)
Other diagnostic tests
Electrical and magnetic stimulation of the abdominal wall muscles are other methods used to quantify the strength of these muscles [25, 79, 81]. As these techniques are cumbersome and uncomfortable, they are rarely used either in clinical practice or for research purposes.
Electromyography of the expiratory muscles has been used in research settings to study the timing of expiratory muscle recruitment during respiration [17, 77], but has not reached clinical implementation. Therefore, these techniques are beyond the scope of this review.
Conclusions
The expiratory muscles are the “neglected component” of the respiratory muscle pump. Rather as the heart does not comprise only a left ventricle, but also a right one, the respiratory muscle pump is much more than just the diaphragm. In this paper, we have summarized the physiology and pathophysiology of expiratory muscles, with a special focus on critically ill patients. Expiratory muscles are frequently recruited in critically ill ventilated patients, but a fundamental understanding of expiratory muscle function is still lacking in these patients. Gastric pressure monitoring provides multiple bedside parameters for analysis of expiratory muscle effort, but their clinical implications need to be established.
References
De Troyer A, Boriek AM (2011) Mechanics of the respiratory muscles. Compr Physiol 1:1273–1300
Dres M, Goligher EC, Heunks LMA, Brochard LJ (2017) Critical illness-associated diaphragm weakness. Intensive Care Med 43:1441–1452
Goligher EC, Fan E, Herridge MS, Murray A, Vorona S, Brace D, Rittayamai N, Lanys A, Tomlinson G, Singh JM, Bolz SS, Rubenfeld GD, Kavanagh BP, Brochard LJ, Ferguson ND (2015) Evolution of diaphragm thickness during mechanical ventilation. impact of inspiratory effort. Am J Respir Crit Care Med 192:1080–1088
Levine S, Nguyen T, Taylor N, Friscia ME, Budak MT, Rothenberg P, Zhu J, Sachdeva R, Sonnad S, Kaiser LR, Rubinstein NA, Powers SK, Shrager JB (2008) Rapid disuse atrophy of diaphragm fibers in mechanically ventilated humans. N Engl J Med 358:1327–1335
Jaber S, Petrof BJ, Jung B, Chanques G, Berthet JP, Rabuel C, Bouyabrine H, Courouble P, Koechlin-Ramonatxo C, Sebbane M, Similowski T, Scheuermann V, Mebazaa A, Capdevila X, Mornet D, Mercier J, Lacampagne A, Philips A, Matecki S (2011) Rapidly progressive diaphragmatic weakness and injury during mechanical ventilation in humans. Am J Respir Crit Care Med 183:364–371
Dres M, Dube BP, Mayaux J, Delemazure J, Reuter D, Brochard L, Similowski T, Demoule A (2017) Coexistence and impact of limb muscle and diaphragm weakness at time of liberation from mechanical ventilation in medical intensive care unit patients. Am J Respir Crit Care Med 195:57–66
van den Berg M, Hooijman PE, Beishuizen A, de Waard MC, Paul MA, Hartemink KJ, van Hees HWH, Lawlor MW, Brocca L, Bottinelli R, Pellegrino MA, Stienen GJM, Heunks LMA, Wust RCI, Ottenheijm CAC (2017) Diaphragm atrophy and weakness in the absence of mitochondrial dysfunction in the critically ill. Am J Respir Crit Care Med 196:1544–1558
Lindqvist J, van den Berg M, van der Pijl R, Hooijman PE, Beishuizen A, Elshof J, de Waard M, Girbes A, Spoelstra-de Man A, Shi ZH, van den Brom C, Bogaards S, Shen S, Strom J, Granzier H, Kole J, Musters RJP, Paul MA, Heunks LMA, Ottenheijm CAC (2018) Positive end-expiratory pressure ventilation induces longitudinal atrophy in diaphragm fibers. Am J Respir Crit Care Med 198:472–485
Heunks L, Ottenheijm C (2018) Diaphragm-protective mechanical ventilation to improve outcomes in ICU patients? Am J Respir Crit Care Med 197:150–152
Schepens T, Dres M, Heunks L, Goligher EC (2019) Diaphragm-protective mechanical ventilation. Curr Opin Crit Care 25:77–85
Jonkman AH, Jansen D, Heunks LM (2017) Novel insights in ICU-acquired respiratory muscle dysfunction: implications for clinical care. Crit Care 21:64
De Troyer A, Ninane V, Gilmartin J, Lemerre C, Estenne M (1987) Triangularis sterni muscle use in supine humans. J Appl Physiol 62:919–925
Wilson TA, Legrand A, Gevenois PA, De Troyer A (2001) Respiratory effects of the external and internal intercostal muscles in humans. J Physiol 530:319–330
De Troyer A, Legrand A, Gevenois PA, Wilson TA (1998) Mechanical advantage of the human parasternal intercostal and triangularis sterni muscles. J Physiol 513(Pt 3):915–925
De Troyer A, Kirkwood PA, Wilson TA (2005) Respiratory action of the intercostal muscles. Physiol Rev 85:717–756
De Troyer A, Estenne M, Ninane V, Van Gansbeke D, Gorini M (1990) Transversus abdominis muscle function in humans. J Appl Physiol (1985) 68:1010–1016
Abe T, Kusuhara N, Yoshimura N, Tomita T, Easton PA (1996) Differential respiratory activity of four abdominal muscles in humans. J Appl Physiol (1985) 80:1379–1389
De Troyer A (1983) Mechanical role of the abdominal muscles in relation to posture. Respir Physiol 53:341–353
Loring SH, Mead J (1982) Abdominal muscle use during quiet breathing and hyperpnea in uninformed subjects. J Appl Physiol Respir Environ Exerc Physiol 52:700–704
Damiani F, Junhasavasdikul D, Dres M, Piraino T, Artigas RM, Chen L, Soliman I, Rauseo M, Rittayamai N, Grieco D, Pham T, Telias IG, Melo L, Friedrich JO, Every H, Greco P, Smith OM, Sandhu G, Gu J, Sinderby CA, Heunks LM, Brochard LJ (2018) Prevalence of reversed triggering assessed by electrical activity of the diaphragm in the first 48 hours of mechanical ventilation. Am J Respir Criti Care Med 197
Aliverti A, Cala SJ, Duranti R, Ferrigno G, Kenyon CM, Pedotti A, Scano G, Sliwinski P, Macklem PT, Yan S (1997) Human respiratory muscle actions and control during exercise. J Appl Physiol (1985) 83:1256–1269
Parthasarathy S, Jubran A, Laghi F, Tobin MJ (2007) Sternomastoid, rib cage, and expiratory muscle activity during weaning failure. J Appl Physiol (1985) 103:140–147
Laghi F, Cattapan SE, Fau-Jubran A, Jubran A, Fau-Parthasarathy S, Parthasarathy S, Fau-Warshawsky P, Warshawsky P, Fau-Choi Y-SA, Choi YS, Fau-Tobin MJ, Tobin MJ (2003) Is weaning failure caused by low-frequency fatigue of the diaphragm? Am J Respir Crit Care Med 167:8
Doorduin J, Roesthuis LH, Jansen D, van der Hoeven JG, van Hees HWH, Heunks LMA (2018) Respiratory muscle effort during expiration in successful and failed weaning from mechanical ventilation. Anesthesiology 129:490–501
Suzuki J, Tanaka R, Yan S, Chen R, Macklem PT, Kayser B (1999) Assessment of abdominal muscle contractility, strength, and fatigue. Am J Respir Crit Care Med 159:1052–1060
Smith J, Bellemare F (1987) Effect of lung volume on in vivo contraction characteristics of human diaphragm. J Appl Physiol (1985) 62:1893–1900
Grimby G, Goldman M, Mead J (1976) Respiratory muscle action inferred from rib cage and abdominal VP partitioning. J Appl Physiol 41:739–751
Derenne JP, Macklem PT, Roussos C (1978) The respiratory muscles: mechanics, control, and pathophysiology. Part 2. Am Rev Respir Dis 118:373–390
Dodd D, Brancatisano T, Engel L (1984) Chest wall mechanics during exercise in patients with severe chronic air-flow obstruction 1–3. Am Rev Respir Dis 129:33–38
Wolfson DA, Strohl KP, Dimarco AF, Altose MD (1983) Effects of an increase in end-expiratory volume on the pattern of thoracoabdominal movement. Respir Physiol 53:273–283
Russell JA, Bishop B (1976) Vagal afferents essential for abdominal muscle activity during lung inflation in cats. J Appl Physiol 41:310–315
Bishop B (1964) Reflex control of abdominal muscles during positive-pressure breathing. J Appl Physiol 19:224–232
McCool FD (2006) Global physiology and pathophysiology of cough: aCCP evidence-based clinical practice guidelines. Chest 129:48S–53S
Langlands J (1967) The dynamics of cough in health and in chronic bronchitis. Thorax 22:88–96
Arora NS, Gal TJ (1981) Cough dynamics during progressive expiratory muscle weakness in healthy curarized subjects. J Appl Physiol Respir Environ Exerc Physiol 51:494–498
Kravitz RM (2009) Airway clearance in Duchenne muscular dystrophy. Pediatrics 123(Suppl 4):S231–S235
Slutsky AS (1999) Lung injury caused by mechanical ventilation. Chest 116:9S–15S
Muscedere JG, Mullen JB, Gan K, Slutsky AS (1994) Tidal ventilation at low airway pressures can augment lung injury. Am J Respir Crit Care Med 149:1327–1334
Talmor D, Sarge T, O’Donnell CR, Ritz R, Malhotra A, Lisbon A, Loring SH (2006) Esophageal and transpulmonary pressures in acute respiratory failure. Crit Care Med 34:1389–1394
Tsuchida S, Engelberts D, Peltekova V, Hopkins N, Frndova H, Babyn P, McKerlie C, Post M, McLoughlin P, Kavanagh BP (2006) Atelectasis causes alveolar injury in nonatelectatic lung regions. Am J Respir Crit Care Med 174:279–289
Guervilly C, Bisbal M, Forel JM, Mechati M, Lehingue S, Bourenne J, Perrin G, Rambaud R, Adda M, Hraiech S, Marchi E, Roch A, Gainnier M, Papazian L (2017) Effects of neuromuscular blockers on transpulmonary pressures in moderate to severe acute respiratory distress syndrome. Intensive Care Med 43:408–418
Pellegrini M, Hedenstierna G, Roneus A, Segelsjo M, Larsson A, Perchiazzi G (2017) The diaphragm acts as a brake during expiration to prevent lung collapse. Am J Respir Crit Care Med 195:1608–1616
Junhasavasdikul D, Telias I, Grieco DL, Chen L, Gutierrez CM, Piraino T, Brochard L (2018) Expiratory flow limitation during mechanical ventilation. Chest 154:948–962
Mead J, Turner J, Macklem P, Little J (1967) Significance of the relationship between lung recoil and maximum expiratory flow. J Appl Physiol 22:95–108
Abdel Kafi S, Serste T, Leduc D, Sergysels R, Ninane V (2002) Expiratory flow limitation during exercise in COPD: detection by manual compression of the abdominal wall. Eur Respir J 19:919–927
Lessard MR, Lofaso F, Brochard L (1995) Expiratory muscle activity increases intrinsic positive end-expiratory pressure independently of dynamic hyperinflation in mechanically ventilated patients. Am J Respir Crit Care Med 151:562–569
Zakynthinos SG, Vassilakopoulos T, Zakynthinos E, Mavrommatis A, Roussos C (2000) Contribution of expiratory muscle pressure to dynamic intrinsic positive end-expiratory pressure: validation using the Campbell diagram. Am J Respir Crit Care Med 162:1633–1640
Vallverdu I, Calaf N, Subirana M, Net A, Benito S, Mancebo J (1998) Clinical characteristics, respiratory functional parameters, and outcome of a two-hour T-piece trial in patients weaning from mechanical ventilation. Am J Respir Crit Care Med 158:1855–1862
Zeggwagh AA, Abouqal R, Madani N, Zekraoui A, Kerkeb O (1999) Weaning from mechanical ventilation: a model for extubation. Intensive Care Med 25:1077–1083
Su WL, Chen YH, Chen CW, Yang SH, Su CL, Perng WC, Wu CP, Chen JH (2010) Involuntary cough strength and extubation outcomes for patients in an ICU. Chest 137:777–782
Savi A, Teixeira C, Silva JM, Borges LG, Pereira PA, Pinto KB, Gehm F, Moreira FC, Wickert R, Trevisan CBE (2012) Weaning predictors do not predict extubation failure in simple-to-wean patients. J Crit Care 27:221.e221–221.e228
Silva CS, Timenetsky KT, Taniguchi C, Calegaro S, Azevedo CS, Stus R, Matos GF, Eid RA, Barbas CS (2012) Low mechanical ventilation times and reintubation rates associated with a specific weaning protocol in an intensive care unit setting: a retrospective study. Clinics (Sao Paulo) 67:995–1000
Kutchak FM, Debesaitys AM, Rieder Mde M, Meneguzzi C, Skueresky AS, Forgiarini Junior LA, Bianchin MM (2015) Reflex cough PEF as a predictor of successful extubation in neurological patients. J Bras Pneumol 41:358–364
Lai CC, Chen CM, Chiang SR, Liu WL, Weng SF, Sung MI, Hsing SC, Cheng KC (2016) Establishing predictors for successfully planned endotracheal extubation. Medicine (Baltimore) 95:e4852
Chao CM, Lai CC, Cheng AC, Chiang SR, Liu WL, Ho CH, Hsing SC, Chen CM, Cheng KC (2017) Establishing failure predictors for the planned extubation of overweight and obese patients. PLoS One 12:e0183360
Hsieh MH, Hsieh MJ, Chen CA-O, Hsieh CC, Chao CM, Lai CC (2018) An artificial neural network model for predicting successful extubation in intensive care units. J Clin Med 7:240. https://doi.org/10.3390/jcm7090240
De Jonghe B, Bastuji-Garin S, Durand M-C, Malissin I, Rodrigues P, Cerf C, Outin H, Sharshar T (2007) Respiratory weakness is associated with limb weakness and delayed weaning in critical illness. Crit Care Med 35:2007–2015
Lanone S, Manivet P, Callebert J, Launay JM, Payen D, Aubier M, Boczkowski J, Mebazaa A (2002) Inducible nitric oxide synthase (NOS2) expressed in septic patients is nitrated on selected tyrosine residues: implications for enzymic activity. Biochem J 366:399–404
Lanone S, Mebazaa A, Heymes C, Henin D, Poderoso JJ, Panis Y, Zedda C, Billiar T, Payen D, Aubier M, Boczkowski J (2000) Muscular contractile failure in septic patients: role of the inducible nitric oxide synthase pathway. Am J Respir Crit Care Med 162:2308–2315
Lanone S, Mebazaa A, Heymes C, Valleur P, Mechighel P, Payen D, Aubier M, Boczkowski J (2001) Sepsis is associated with reciprocal expressional modifications of constitutive nitric oxide synthase (NOS) in human skeletal muscle: down-regulation of NOS1 and up-regulation of NOS3. Crit Care Med 29:1720–1725
Lanone S, Taillé C, Boczkowski J, Aubier M (2012) Diaphragmatic fatigue during sepsis and septic shock. Appl Physiol Intensive Care Med 1:309–315
Hooijman PE, Beishuizen A, Witt CC, de Waard MC, Girbes AR, Spoelstra-de Man AM, Niessen HW, Manders E, van Hees HW, van den Brom CE, Silderhuis V, Lawlor MW, Labeit S, Stienen GJ, Hartemink KJ, Paul MA, Heunks LM, Ottenheijm CA (2015) Diaphragm muscle fiber weakness and ubiquitin-proteasome activation in critically ill patients. Am J Respir Crit Care Med 191:1126–1138
American Thoracic Society/European Respiratory S (2002) ATS/ERS Statement on respiratory muscle testing. Am J Respir Crit Care Med 166:518–624
Savi A, Teixeira C, Silva JM, Borges LG, Pereira PA, Pinto KB, Gehm F, Moreira FC, Wickert R, Trevisan CB, Maccari JG, Oliveira RP, Vieira SR, Gaucho Weaning Study G (2012) Weaning predictors do not predict extubation failure in simple-to-wean patients. J Crit Care 27:221–228
Friedrich O, Reid MB, Van den Berghe G, Vanhorebeek I, Hermans G, Rich MM, Larsson L (2015) The sick and the weak: neuropathies/myopathies in the critically ill. Physiol Rev 95:1025–1109
Petrof BJ, Hussain SN (2016) Ventilator-induced diaphragmatic dysfunction: what have we learned? Curr Opin Crit Care 22:67–72
Aliverti A, Carlesso E, Dellaca R, Pelosi P, Chiumello D, Pedotti A, Gattinoni L (2006) Chest wall mechanics during pressure support ventilation. Crit Care 10:R54
Malbrain ML, Roberts DJ, De Laet I, De Waele JJ, Sugrue M, Schachtrupp A, Duchesne J, Van Ramshorst G, De Keulenaer B, Kirkpatrick AW, Ahmadi-Noorbakhsh S, Mulier J, Ivatury R, Pracca F, Wise R, Pelosi P (2014) The role of abdominal compliance, the neglected parameter in critically ill patients—a consensus review of 16. Part 1: definitions and pathophysiology. Anaesthesiol Intensive Ther 46:392–405
Laghi F, Tobin MJ (2003) Disorders of the respiratory muscles. Am J Respir Crit Care Med 168:10–48
Tobin MJ, Laghi F, Jubran A (2010) Narrative review: ventilator-induced respiratory muscle weakness. Ann Intern Med 153:240–245
Schellekens WJ, van Hees HW, Doorduin J, Roesthuis LH, Scheffer GJ, van der Hoeven JG, Heunks LM (2016) Strategies to optimize respiratory muscle function in ICU patients. Crit Care 20:103
Dres M, Goligher EC, Heunks LMA, Brochard LJ (2017) Critical illness-associated diaphragm weakness. Intensive Care Med 43:1441–1452
Man WD, Kyroussis D, Fleming TA, Chetta A, Harraf F, Mustfa N, Rafferty GF, Polkey MI, Moxham J (2003) Cough gastric pressure and maximum expiratory mouth pressure in humans. Am J Respir Crit Care Med 168:714–717
Rooban N, Regli A, Davis WA, De Keulenaer BL (2012) Comparing intra-abdominal pressures in different body positions via a urinary catheter and nasogastric tube: a pilot study. Ann Intensive Care 2(Suppl 1):S11
Norisue Y, Kataoka J, Homma Y, Naito T, Tsukuda J, Okamoto K, Kawaguchi T, Ashworth L, Yumiko S, Hoshina Y, Hiraoka E, Fujitani S (2018) Increase in intra-abdominal pressure during airway suctioning-induced cough after a successful spontaneous breathing trial is associated with extubation outcome. Ann Intensive Care 8:61
Appendini L, Patessio A, Zanaboni S, Carone M, Gukov B, Donner CF, Rossi A (1994) Physiologic effects of positive end-expiratory pressure and mask pressure support during exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 149:1069–1076
Parthasarathy S, Jubran A, Tobin MJ (1998) Cycling of inspiratory and expiratory muscle groups with the ventilator in airflow limitation. Am J Respir Crit Care Med 158:1471–1478
Jubran A, Van de Graaff WB, Tobin MJ (1995) Variability of patient-ventilator interaction with pressure support ventilation in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 152:129–136
Polkey MI, Luo Y, Guleria R, Hamnegard CH, Green M, Moxham J (1999) Functional magnetic stimulation of the abdominal muscles in humans. Am J Respir Crit Care Med 160:513–522
Hamnegard CH, Wragg S, Kyroussis D, Mills GH, Polkey MI, Moran J, Road J, Bake B, Green M, Moxham J (1996) Diaphragm fatigue following maximal ventilation in man. Eur Respir J 9:241–247
Kyroussis D, Mills GH, Polkey MI, Hamnegard CH, Koulouris N, Green M, Moxham J (1996) Abdominal muscle fatigue after maximal ventilation in humans. J Appl Physiol (1985) 81:1477–1483
Bai TR, Rabinovitch BJ, Pardy RL (1984) Near-maximal voluntary hyperpnea and ventilatory muscle function. J Appl Physiol Respir Environ Exerc Physiol 57:1742–1748
Kyroussis D, Polkey MI, Hamnegard CH, Mills GH, Green M, Moxham J (2000) Respiratory muscle activity in patients with COPD walking to exhaustion with and without pressure support. Eur Respir J 15:649–655
Cabello B, Mancebo J (2006) Work of breathing. Intensive Care Med 32:1311–1314
Mauri T, Yoshida T, Bellani G, Goligher EC, Carteaux G, Rittayamai N, Mojoli F, Chiumello D, Piquilloud L, Grasso S, Jubran A, Laghi F, Magder S, Pesenti A, Loring S, Gattinoni L, Talmor D, Blanch L, Amato M, Chen L, Brochard L, Mancebo J, Group PLpw (2016) Esophageal and transpulmonary pressure in the clinical setting: meaning, usefulness and perspectives. Intensive Care Med 42:1360–1373
Vassilakopoulos T (2008) Understanding wasted/ineffective efforts in mechanically ventilated COPD patients using the Campbell diagram. Intensive Care Med 34:1336–1339
Ninane V, Rypens F, Yernault J-C, De Troyer A (1992) Abdominal muscle use during breathing in patients with chronic airflow obstruction1″ 3. Am Rev Respir Dis 148:16–21
Condessa RL, Brauner JS, Saul AL, Baptista M, Silva AC, Vieira SR (2013) Inspiratory muscle training did not accelerate weaning from mechanical ventilation but did improve tidal volume and maximal respiratory pressures: a randomised trial. J Physiother 59:101–107
Fregonezi G, Azevedo IG, Resqueti VR, De Andrade AD, Gualdi LP, Aliverti A, Dourado MET, Parreira VF (2015) Muscle impairment in neuromuscular disease using an expiratory/inspiratory pressure ratio. Respir Care 60:533–539
Gobert F, Yonis H, Tapponnier R, Fernandez R, Labaune MA, Burle JF, Barbier J, Vincent B, Cleyet M, Richard JC, Guerin C (2017) Predicting extubation outcome by cough peak flow measured using a built-in ventilator flow meter. Respir Care 62:1505–1519
Goligher EC, Laghi F, Detsky ME, Farias P, Murray A, Brace D, Brochard LJ, Bolz SS, Rubenfeld GD, Kavanagh BP, Ferguson ND (2015) Measuring diaphragm thickness with ultrasound in mechanically ventilated patients: feasibility, reproducibility and validity. Intensive Care Med 41:642–649
Matamis D, Soilemezi E, Tsagourias M, Akoumianaki E, Dimassi S, Boroli F, Richard JC, Brochard L (2013) Sonographic evaluation of the diaphragm in critically ill patients. Technique and clinical applications. Intensive Care Med 39:801–810
McMeeken JM, Beith ID, Newham DJ, Milligan P, Critchley DJ (2004) The relationship between EMG and change in thickness of transversus abdominis. Clin Biomech (Bristol, Avon) 19:337–342
Misuri G, Colagrande S, Gorini M, Iandelli I, Mancini M, Duranti R, Scano G (1997) In vivo ultrasound assessment of respiratory function of abdominal muscles in normal subjects. Eur Respir J 10:2861–2867
Rankin G, Stokes M, Newham DJ (2006) Abdominal muscle size and symmetry in normal subjects. Muscle Nerve 34:320–326
Tahan N, Khademi-Kalantari K, Mohseni-Bandpei MA, Mikaili S, Baghban AA, Jaberzadeh S (2016) Measurement of superficial and deep abdominal muscle thickness: an ultrasonography study. J Physiol Anthropol 35:17
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Shi, ZH., Jonkman, A., de Vries, H. et al. Expiratory muscle dysfunction in critically ill patients: towards improved understanding. Intensive Care Med 45, 1061–1071 (2019). https://doi.org/10.1007/s00134-019-05664-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-019-05664-4