Abstract
Purpose
Hepatocellular carcinoma (HCC) poses a unique challenge due to its predilection for developing on compromised livers, often limiting surgical options. Stereotactic body radiotherapy (SBRT) has emerged as a promising local treatment modality for HCC. This study aims to assess the effectiveness of SBRT in HCC patients not suitable for surgery, focusing on local control, optimal radiation dosing, and prognostic factors.
Methods
In this retrospective analysis, 52 HCC patients treated with SBRT were examined. The study assessed local control, progression-free survival (PFS), and overall survival (OS) while conducting dosimetric analyses. The relationship between mean liver dose and Child–Pugh score (CPS) progression was also explored.
Results
SBRT demonstrated 93.4% freedom from local progression (FFLP) at 12 months. Notably, a near minimum dose (D98%) below 61 Gy as an equivalent dose in 2‑Gy fractions with α/β 10 Gy (EQD2α/β10) was associated with reduced FFLP (p-value 0.034). Logistic regression analysis revealed a dose–response relationship for FFLP and D98% with 95% and 98% probability of FFLP at a dose of 56.9 and 73.1 Gy, respectively. The study observed OS rates of 63.7% at 1 year and 34.3% at 3 years. Patients with portal vein tumor thrombus (PVTT) and larger tumors (≥ 37 cm3) experienced decreased PFS and OS. Multivariate analysis identified PVTT, larger tumor volume, and performance status as independent predictors of reduced OS. Notably, classical radiation-induced disease (cRILD) was absent, but nonclassical (nc) RILD occurred in 7.7% of patients. Regression analysis linked a mean EQD2α/β3–8 dose to the liver (12.8–12.6) with a 10% likelihood of ncRILD.
Conclusion
SBRT offers a compelling option for achieving high local control and promising survival outcomes in HCC. The study supports a radiation dose range of 61–73.1 Gy, coupled with a mean liver dose under 12.6–12.8 Gy as EQD2, to achieve favorable FFLP rates, with acceptable toxicity rates.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Liver cancer, ranking as the 6th most prevalent malignancy globally and standing as the third leading cause of cancer-related death, is a challenging malignancy and is surpassed only by lung and colorectal cancer [1]. Hepatocellular carcinoma (HCC) is the most common primary liver cancer, accounting for > 80% of cases [2]. Traditionally, surgical resection and orthotopic liver transplantation have been considered the cornerstone of curative interventions for HCC, delivering survival rates exceeding 5 years in cases of early stage HCC [3]. However, their applicability is restricted by stringent criteria. A common predicament is the unsuitability of certain patients for surgical intervention, primarily due to poor hepatic reserve, concurrent comorbidities, or presenting in an advanced stage [4].
In response to these limitations, locoregional therapies such as radiofrequency ablation (RFA), microwave ablation (MWA), or transarterial arterial chemoembolization (TACE) have been employed as minimally invasive alternatives in early and intermediate stages [3].
Meanwhile, the recent advancement in radiation therapy technology has enabled the precise delivery of lethal high radiation doses to localized tumors in one or a few fractions [5]. Consequently, SBRT has gained widespread acceptance as a treatment modality for various primary tumors, including those in the lungs, prostate, liver, and pancreas, or as an ablative tool for various metastatic sites in oligometastatic disease [6,7,8,9,10]. An expanding body of evidence supports the safe and effective application of SBRT in diverse stages in HCC, including early, intermediate, and advanced stages or in the setting of planned orthoptic liver transplantation as a bridging therapy, yielding impressive high control rates of 70–100% [8, 11,12,13,14,15,16].
Nevertheless, ongoing debates regarding the use of SBRT in HCC warrant further exploration. These include determining the optimal radiation dose that can achieve effective local control while minimizing additional radiation exposure to the cirrhotic liver tissue. In addition, there is a need to identify specific patient subsets that may derive substantial benefit from the high local control rates offered by SBRT and those subsets at higher risk for hepatic and extrahepatic progression who might need or benefit more from combined therapeutic approaches.
In the current retrospective study, we aimed to report on the single institution experience of stereotactic body radiotherapy in HCC, emphasizing local control outcomes as well as the overall and progression-free survivals. Additionally, we aim to determine the optimal radiation dose for HCC that achieves a high control rate while maintaining an acceptably low toxicity profile for SBRT. Finally, we also aimed to investigate the prognostic factors that influence survival to enable us to define suitable candidates for combined therapies in the future.
Materials and methods
Following approval from the local ethics committee (RWTH Aachen University, Faculty of Medicine, EK 23–264), we conducted a retrospective analysis of patients diagnosed with HCC who had undergone SBRT as part of their disease management between January 2013 and June 2023. Each patient’s case was discussed in a multidisciplinary tumor board comprising experts from various disciplines, including hepatology, medical oncology, hepatobiliary and transplant surgery, interventional and diagnostic radiology, and radiation oncology. Nonsurgical local treatments, including SBRT, RFA, MWA, TACE, and selective internal radiation therapy (SIRT), were offered to patients deemed ineligible for surgical resection, still suitable for local therapy. The choice of SBRT over other treatment options was based on specific factors such as tumor location, including those near the liver dome or blood vessels, lesion size exceeding 3 cm, contraindications for anesthesia, and the need for adjuvant or salvage therapy following TACE.
Only patients who received liver-directed SBRT, as defined by the German Society for Radiation Oncology [5], were included in the analysis. Exclusion criteria were (1) radiation was delivered in symptom palliation, (2) SBRT to lesion outside the liver, (3) none of the survival parameters could be retrieved, and (4) active second malignancy at the time of SBRT application.
A cohort of 54 patients diagnosed with HCC who underwent liver-directed SBRT as part of their disease management was initially identified. However, 2 patients were subsequently excluded from the analysis: one patient was undergoing active treatment for non-small cell lung cancer at the time of SBRT delivery, and no retrievable survival data were accessible for the second patient. Therefore, our final analysis encompassed a total of 52 patients. Among them, comprehensive survival and imaging data from 49 patients, including a total of 62 treated lesions, were available for this study. For the remaining 3 patients, only overall survival data could be analyzed.
SBRT planning and delivery
First, patients received coaching for inspiration breath-hold (iBH). If patients sustained iBH for 20–30 s, they were considered eligible for simulation and treatment in iBH; if not, they were considered for 4D simulation and treatment.
Briefly, each patient received computer tomography for planning purposes (P-CT) on a 16-slice CT scanner (Brilliance CT Big Bore Oncology, Philips Medical Systems, Inc., Cleveland, OH, USA) in vacuum cushions. Patients received screen goggles connected to an optical surface scanning system (CRAD, Uppsala, Sweden) during the P‑CT and radiation session to facilitate regular breathing in the target zone.
Patients received the three phases of contrast medium-enhanced CT (early arterial, venous, and late venous phases) in the same breathing phase/phases of the P‑CT. If fiducials were necessary, the insertion was applied CT or ultrasound-guided 1 week before P‑CT.
Planning and diagnostic scans were transferred to the planning system (Pinnacle, V.14.0, Philips Healthcare, Amsterdam, The Netherlands). In the case of radiation intrahepatic lesions, gross tumor volume (GTV) was defined as lesions with arterial enhancement and venous washout. In the case of radiation to portal vein tumor thrombus (PVTT), the whole thrombus was considered as GTV, irrespective of enhancing the characteristics. Internal target volume (ITV) was applied for patients who received a 4D CT. In short, contrast-enhanced CTs were deformable, registered with 0%, 50%, and 90% respiratory phase-CTs, and ITV was the sum of arterial enhancement of all three phases. Clinical target volume (CTV) was considered in certain situations as previous TACE of the lesion (including the whole nonenhancing part of the lesion as well) or in the case of SBRT of the recurrent lesion after surgical resection (including the entire resection cavity as well). Planning target volume (PTV) was generated by adding isotopic margins of 5 mm to GTV/ITV/CTV.
The radiation dose was prescribed to enclose PTV in 80% isodose-line to generate the required dose inhomogeneity inside the target volume. Planning aimed to ensure the best coverage of the target volume without compromising the constraints of organs at risk (supplementary table 1), underdosage in the target volume was allowed to ensure the constraints for organs at risk were met. SBRT was delivered as flattening filter-free (FFF) volumetric modulated arc therapy (VMAT). Radiotherapy was delivered 3–4 times weekly as image-guided radiation therapy (IGRT) using cone-beam CT imaging (XVI, Elekta, Stockholm, Sweden) before each fraction for accurate radiation delivery.
Outcomes evaluation
One month after SBRT, patients were assessed clinically and serologically; this included a complete blood count (CBC), liver function test (LFT), and alpha-fetoprotein (AFP). Imaging assessment using CT or MRI followed 3 months (2 months in case PVTT) after SBRT, and subsequently, every 3 months for 24 months afterward, and finally every 6 months if there were no signs of progression. The radiological response assessment was evaluated based on the modified Response Evaluation Criteria in Solid Tumors (RECIST) criteria for HCC [17]. In summary, complete response (CR) was defined by the complete disappearance of the initial irradiated contrast-enhanced lesion in the arterial phase or tumor disappearance for PVTT (the treated lesion), partial response (PR) was defined as a more than 30% reduction in the size of the treated lesion, stable disease (SD) was defined as reduction size of the treated lesion < 30% or light increase in the size < 20%, and progressive disease (PD) was defined as having an increase of 20% in the size of the treated lesion.
The toxicity was graded using the National Cancer Institute Common Terminology Criteria for Adverse Events (CTAE) version 5.
Radiation-induced liver disease (RILD) is a not-infrequent complication of liver-directed SBRT. For the analysis, we evaluated both forms of RILD. The classical form, cRILD, was defined as anicteric hepatomegaly with ascites and elevated serum alkaline phosphatase more than twice the baseline or upper limit of the normal [18]. The nonclassical form of RILD (ncRILD) was defined as Child–Pugh score (CPS) progression ≥ 2 points or elevation of the alanine transaminases more than four times the upper limit [19].
Statistical analysis
The primary endpoint of the analysis was freedom from local progression (FFLP), defined at the treated lesion level as the time from radiation initiation until the subsequent local progression or censored. Overall survival (OS) was defined as the interval from the initiation of SBRT to the time of death or censoring. Progression-free survival (PFS) was defined as the interval from initiating the radiation treatment to the point of any site disease progression or censoring. In the case of a liver transplant, FFLP for the respective patient was censored at the time of transplant.
The log-rank test was used for univariate analysis, and the Cox regression was used for independent predictors for PFS and OS. The time-dependent receiver operating characteristic curve (ROC) analysis was applied to calculate the most robust statistical significance cut-point. Mathematical models for FFLP using logistic regression to estimate FFLP probability as a function of prescribed dose (PD) and D98% were created using the radiation dose as EQD2α/β10.
where
-
P (Y = 1) is the probability of the event occurring (tumor control),
-
e is the base of the natural logarithm,
-
β0 is the intercept term in the logistic regression equation, and
-
β1 is the coefficient for the independent variable X (radiation dose).
The same was applied for calculating the probability of ncRILD using logistic regression analysis between the mean radiation dose to the liver into EQD2α/β3 and EQD2α/β8 and CPS progression ≥ 2 points.
The statistical analysis and graphics were executed using the R software version 4.3.1.
Results
Table 1 provides an overview of patient and disease characteristics. The median follow-up period for the entire cohort was 16.7 months. Sixteen patients had early-stage HCC, and 36 had intermediate and advanced stages (Barcelona clinic liver cancer “BCLC” stage B and C), with 25 patients with BCLC B and C having received prior therapy before SBRT. Sixty-two lesions were evaluable for the analysis with a median volume of 8.15 (0.5–539) cm3. The median of the prescribed physical dose was 40 Gy in a median 5 fractions (EQD2α/β10 60 Gy).
Notably, 4 patients received SBRT as bridging for a liver transplant and were subsequently transplanted at 1.5, 2.4, 6.2, and 10 months after SBRT. Three patients had a pathological complete response (pCR) at the time of the transplant (2.4, 6.2, and 10 months after SBRT), and 1 patient had a pathological partial response (viable cells in 20% of the lesion 1.5 months after SBRT), the survival of the 4 patients was 9, 10.5, 12.3 and 17.7 months at the time of the analysis (supplementary figure 1).
Local tumor control
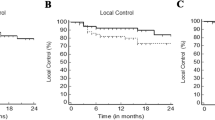
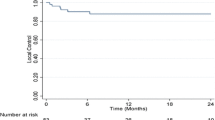
Of the 62 lesions treated with SBRT in 49 patients, the response pattern was as follows: 40 lesions displayed complete responses (CR) at a rate of 64.5%, 10 exhibited partial responses (PR) at 16%, 9 remained in a stable disease (SD) state at 14.5%, and 3 displayed progressive disease (PD) at 5%. In total, four lesions encountered local progression; three experienced direct radiographic local progressions at 4.9, 5.3, and 7 months posttreatment, while one lesion initially showed CR; however, a new lesion or lesion regrowth occurred in the former PTV 27.3 months after treatment. FFLP rates at 12, 24, and 36 months were 93.4%, 93.4%, and 80.1%, respectively (Fig. 1a).
Kaplan–Meier curve showing a freedom from local progression (FFLP) for the irradiated lesions, b difference in FFLP for lesions with prescribed dose (PD) EQD2α/β10 ≥ 61 Gy and < 61 Gy, and c the difference in FFLP for lesions with dose near minimum (D98%) EQD2α/β10 ≥ 61 Gy and < 61 Gy, *p-value < 0.05: statistically significant, log rank test. d FFLP model for irradiated hepatocellular carcinoma (HCC) lesions, the logistic regression curve describes the relationship between local control in 3 years and the dose near minimum (D98) EQD2α/β10, purple and green dotted lines represent the radiation dose that achieves 95%and 98% FFL, respectively
Univariate analysis was conducted to investigate the influence of tumor size, the treatment of intrahepatic lesions versus PVTT on local control, and dosimetric parameters of the SBRT. No statistically meaningful differences existed between small and larger tumors (< 37 vs ≥ 37 cm3) or intrahepatic versus PVTT in FFLP. Furthermore, the prescribed radiation dose (PD) in equivalent dose in 2‑Gy fractions (EQD2α/β10) to the planning target volume (PTV) below 61 Gy exhibited a trend for association with lower FFLP (P-value 0.078; Fig. 1b). Also, examining D98% of PTV showed a D98% < 61 Gy (EQD2α/β10), associated with a statistically significant lower FFLP (P-value 0.034; Fig. 1c).
In addition, a logistic regression analysis was performed to examine the relationship between dosimetric parameters of the PTV and FFLP.
The initial model, examining PD as a predictor, demonstrated a modest dose–response relationship with the probability of FFLP. The estimated dose coefficient (β1) was 0.043 with a corresponding p-value of 0.35, suggesting a limited statistical significance. Conversely, the second model, which considered D98% for PTV as a predictor, indicated a more substantial dose–response relationship, β1 was noted at 0.06 with a p-value of 0.085, approaching statistical significance. Using this model, the projected dose to achieve 95 and 98% FFLP were at 56.9 and 73.1 Gy, respectively (Fig. 1d).
Survival outcomes
In all, 17, 3, and 4 patients experienced hepatic, extrahepatic, and hepatic and extrahepatic progressions, respectively, as the first site of progression after SBRT at the time of the analysis with median PFS was 11.4 months (Fig. 2b); a subsequent SBRT after disease progression was applied in 5 patients, TACE in 5 patients, RFA in 2 patients, SIRT in 1 patient, and systemic treatment in 12 patients. The median PFS for stage A was not reached at the time of the analysis, and for stages B and C were 10.9 and 4.9 months, respectively (Fig. 2c).
Kaplan–Meier curve shows the progression-free survival (PFS; a) and overall survival (OS; b) for the entire cohort. Univariate analysis using Kaplan–Meier curves (KM) with log-rank test for PFS and OS based on disease stage (BCLC; c, d), irradiation of portal vein tumor thrombus (PVTT; e, f), tumor volume ≥ 37 cm3 (g, h), alpha-fetoprotein level > 23 ng/ml (i, j). *p-value < 0.05: statistically significant, log-rank test
Twenty-three out of 52 patients had passed away at the time of the analysis, with a median OS of 29.3 months. The OS at 1 and 3 years were 63.7 and 34.3%, respectively (Fig. 2a). The median OS for stage A was not reached, while the median OS for stage B and C was 29.3 and 7.1 months, respectively (Fig. 2c).
A cut-off point for tumor volume (37 cm3) and AFP (23 ng/ml) was identified using ROC analysis. In the univariate analysis, SBRT of PVTT or lesions ≥ 37 cm3 were associated with statistically significant shorter PFS (p-value 0.00025 and 0.032 respectively) and OS (p-value 0.002 and 0.008, respectively; Fig. 2c, d, h, i, Table 2). Furthermore, pre-SBRT AFP > 23 ng/ml was associated with PFS (P-value 0.026); however, this was not translated into a difference in OS (p-value 0.54; Fig. 2e, j, Table 2). Postprogression TACE or systemic therapy did not alter OS compared to those who did not receive those interventions (p-value 0.13 and 0.65, respectively, supplementary figure 2; Table 2).
In the multivariant analysis using Cox regression, a model using ECOG status, PVTT, tumor volume, and AFP for PFS and OS was conducted based on the univariate analysis; the C‑index for the model was 0.72 and 0.75, respectively. PVTT (hazard ratio [HR] 4.04) and tumor volume ≥ 37 cm3 (HR 2.62) were associated with shorter PFS. Nonetheless, ECOG status (HR 4.5), tumor volume ≥ 37 cm3 (HR 3.7), and PVTT (HR 3.5) were associated with shorter OS (Table 2).
Change in Child–Pugh score and severe toxicity
CRILD occurred in none of the patients under investigation. However, 4 patients (7.7%) encountered a permanent one-point increase in CPS within the 6 months following SBRT. Conversely, 1 patient (1.9%) experienced a one-point improvement in their CP score after undergoing SBRT. Additionally, 4 patients (7.7%) experienced a two-point progression in CPS within 6 months after SBRT (ncRILD) Tragically, 1 patient (1.9%) with a two-point CPS progression passed away due to hemorrhagic shock resulting from refractory variceal bleeding 3 months after SBRT.
Further, we performed a logistic regression analysis between the liver mean dose (EQD2α/β3&8) and CP score two-point progression (ncRILD) at 6 months [20]. β1 for the model using EQD2α/β3 was 0.15 and approaching the statistical significance (p-value 0.06), while β1 for model with EQD2α/β8 was 0.17 and statistically significant (p-value 0.048). The analysis revealed a 10% probability of a two-point progression in CPS at 12.8 Gy (EQD2α/β3) and 12.6 (EQD2α/β8) mean dose to the liver, with an exponential increase in the probability of two-point CPS progression after 15 Gy, reaching an almost 50% probability at 25 Gy (EQD2α/β3; Fig. 3). The mean dose delivered to the liver for patients with two-point progression was not statistically significantly higher than for patients with stable or one-point CP progression (suppl. figure 2).
None of the patients experienced biliary stenosis or grade 3 or higher gastrointestinal toxicities.
Discussion
Previously, the treatment results of HCC with radiation were unsatisfactory due to the limited accuracy in the application of the radiation dose to the target volume, which was compensated with the irradiation of a large volume of healthy liver parenchyma, resulting in high toxicity and a lower response rate [21]. Lately, stereotactic radiotherapy (SRT), initially developed for treating cranial tumors, emerged as one of the curative methods for early HCC, delivering ablative radiation doses to tumors while sparing the adjacent healthy liver tissues and yielding a high local control rate of around 98–90% [22,23,24]. Comparative studies with thermal ablation showed similar efficacy for small lesions and better results for tumors larger than 3 cm in diameter or the subphrenic area of the liver [15, 25, 26]. Nonetheless, several retrospective and prospective studies showed the superiority of SBRT regarding the local control for HCC compared to TACE [27,28,29,30].
The current analysis provides some insights into managing HCC using SBRT. The assessment of local tumor control is paramount when evaluating the efficacy of any local treatment modality for HCC. In this study, the local tumor control achieved with SBRT was convincingly high, with FFLP rates at 1, 2 and 3 years of 93.4%, 93.4%, and 80.1%, respectively, with local progression in only 4 cases, underscoring the potential of SBRT in achieving durable local control. In the univariate analysis, the tumor volume, or intrahepatic tumors versus PVTT did not differ for the local control. Notably, PD as EQD2α/β10 ≥ 61 Gy showed a trend for better local control (p-value 0.078), and D98 as EQD2α/β10 > 61 was associated with a statistically significant improvement of local FFLP (p-value 0.034).
Previously, various studies tried to find a dose–response relationship in HCC; some could not establish one [31], while others claimed an advantage for higher doses over lower ones for the local control [32, 33]. In our analysis, the logistic regression model relating PD to FFLP showed a weak dose–response relationship, with β1 of 0.043 and a nonsignificant p-value “0.35”. This suggests a limited direct correlation between PD in the reported dose range (median 40 Gy in 5 fractions: EQD2α/β10 : 60 Gy, interquartile range 56–70 Gy) and local tumor control, a finding that aligns with Ohri et al. [31]. The analysis involving D98% presented a more pronounced dose–response curve, with a β1 of 0.06 and a marginally significant p-value “0.085” with TCP values for 95 and 98%, projected at 56.9 and 73.1 Gy, respectively. This enhanced relationship suggests that D98% may serve as a more reliable predictor for local tumor control, potentially providing a more precise dosimetric criterium for the evaluation of the radiation therapy plan in HCC. The discrepancy in the predictive value of PD and D98% could be attributed to the PD parameter not accounting for the spatial distribution of the dose within the PTV, unlike D98%, which is more reflective of the actual dose coverage in the target volume. These considerations are pivotal and may underscore the implication of an individualized dose prescription in radiation therapy for HCC. Based on the current analysis, an EQD2α/β10 ≥ 61 Gy (equal to biological effective dose “BEDα/β10” 73.3 Gy) proved to be sufficient to reach a high probability of tumor control, but a higher EQD2α/β10 > 73.1 Gy (BEDα/β10 85 Gy) may not translate into a meaningful advantage for FFLP.
These results should be considered in the light of the assessment of the treatment-related toxicity, which is a critical aspect of any therapeutic intervention. In this study, cRILD was not observed, suggesting the safety of SBRT to spare the uninvolved liver parenchyma. However, changes in CPS were noted; namely, 4 patients (7.7%) experienced meaningful CPS progression ≥ 2 points (ncRILD), and 1 of these patients (1.9%) died of refractory variceal bleeding after CPS progression. Dawson et al. suggested that the dose mean to the liver is the most useful parameter in predicting cRILD in liver SBRT, with cRILD not observed when the dose mean to the liver is below 31 Gy [34]. Nonetheless, ncRILD is mostly underestimated in SBRT of HCC, with some studies reporting its incidence around 20% and suggesting a dose mean to liver below 15 Gy to reduce its incidence [35, 36]. We further generated a logistic regression model to predict ncRILD using the dose mean to the liver. The logistic regression model showed a 10% probability of ncRILD at 12.8 and 12.6 Gy, as EQD2α/β3 and EQD2α/β8, respectively. It is important to note that the dose mean to the liver for patients with CPS B and C was further kept below 8 Gy, with few exceptions at the initial phase of our SBRT experience.
Regarding survival outcomes, the median OS for patients with “BCLC A” was not reached at the time of the analysis, while the “BCLC B” and “C” were 29.3 and 7.1 months, respectively, despite the high local control rate for the treated lesions. We investigated the possible tumor characteristics associated with worse PFS and OS. Predictably, advanced CP score (B and C) or performance status were associated with reduced OS. Also, the presence of PVTT or a larger tumor ≥ 37 cm3 strongly predicted shorter PFS and OS. These results may be useful in a number of aspects, such as the proper selection of patients with good performance status who profit from local therapy. Further, the local therapy tool, such as SBRT, alone may not be sufficient for larger tumors or PVTT to achieve intra- and extrahepatic tumor control [37], and a possible combination for these stages (BCLC B or C) with systemic therapy could be advantageous. Indeed, NRG 11112 recently showed that combining SBRT with sorafenib for stage BCLC B and C resulted in a durable response with higher OS and PFS than sorafenib alone [38]. Novel combinations with immunotherapy are currently being investigated with promising results [39].
Also, the utilization of repeated SBRT after hepatic progression was feasible in 5 patients without increased toxicities and aligns with similar previous reports [40].
Study limitations
There are some limitations of the current study that should be addressed. The main limitation is the retrospective nature of the study, which could make the analysis prone to selection bias and confounding factors that may impact the results. Second, the patient cohort includes heterogenous tumor stages, and a relatively limited number of patients for each stage group implies caution when addressing the study results.
Conclusion
This study demonstrates the potential of stereotactic body radiotherapy (SBRT) as an effective treatment modality for hepatocellular carcinoma (HCC), achieving promising local control rates with manageable toxicity across different stages of HCC. SBRT with EQD2α/β10 61–73.1 Gy may be sufficient to achieve durable local control and avoid unnecessary radiation dose exposure to the liver. The dose mean to the liver is the main parameter to predict the Child–Pugh score (CPS) progression after SBRT and should be held below 12.8–12.6 Gy (EQD2α/β3–8) and may be lower for CPS B and C. Finally, tumors ≥ 37 cm3 and portal vein tumor thrombus (PVTT) may profit more from combining the SBRT with systemic therapy to delay the intra- and extrahepatic progression. Current prospective studies are investigating such combinations.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71(3):209–249
El-Serag HB, Rudolph KL (2007) Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology 132(7):2557–2576
Galle PR, Forner A, Llovet JM, Mazzaferro V, Piscaglia F, Raoul JL, Schirmacher P, Vilgrain V (2018) EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 69(1):182–236. https://doi.org/10.1016/j.jhep.2018.03.019
Kudo M, Izumi N, Kokudo N, Sakamoto M, Shiina S, Takayama T, Tateishi R, Nakashima O, Murakami T, Matsuyama Y et al (2021) Report of the 21st nationwide follow-up survey of primary liver cancer in Japan (2010–2011). Hepatol Res 51(4):355–405
Guckenberger M, Baus WW, Blanck O, Combs SE, Debus J, Engenhart-Cabillic R, Gauer T, Grosu AL, Schmitt D, Tanadini-Lang S et al (2020) Definition and quality requirements for stereotactic radiotherapy: consensus statement from the DEGRO/DGMP working group stereotactic radiotherapy and radiosurgery. Strahlenther Onkol 196(5):417–420. https://doi.org/10.1007/s00066-020-01603-1
Palma DA, Olson R, Harrow S, Gaede S, Louie AV, Haasbeek C, Mulroy L, Lock M, Rodrigues GB, Yaremko BP et al (2019) Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): a randomised, phase 2, open-label trial. Lancet 393(10185):2051–2058. https://doi.org/10.1016/S0140-6736(18)32487-5
Gkika E, Adebahr S, Kirste S, Schimek-Jasch T, Wiehle R, Claus R, Wittel U, Nestle U, Baltas D, Grosu AL et al (2017) Stereotactic body radiotherapy (SBRT) in recurrent or oligometastatic pancreatic cancer. Strahlenther Onkol 193(6):433–443. https://doi.org/10.1007/s00066-017-1099-8
Bujold A, Massey CA, Kim JJ, Brierley J, Cho C, Wong RKS, Dinniwell RE, Kassam Z, Ringash J, Cummings B et al (2013) Sequential phase I and II trials of stereotactic body radiotherapy for locally advanced hepatocellular carcinoma. J Clin Oncol 31(13):1631–1639
Chang JY, Senan S, Paul MA, Mehran RJ, Louie AV, Balter P, Groen HJM, McRae SE, Widder J, Feng L et al (2015) Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Oncol 16(6):630–637
Mohamed AA, Goncalves M, Singh BP, Tometten M, Rashad A, Hölzle F, Hackenberg S, Eble M (2024) Stereotactic radiotherapy in the management of oligometastatic and recurrent head and neck cancer: a single-center experience. Strahlenther Onkol. https://doi.org/10.1007/s00066-023-02180-9
Hara K, Takeda A, Tsurugai Y, Saigusa Y, Sanuki N, Eriguchi T, Maeda S, Tanaka K, Numata K (2019) Radiotherapy for hepatocellular carcinoma results in comparable survival to radiofrequency ablation: a propensity score analysis. Hepatology. https://doi.org/10.1002/hep.30591
Kimura T, Aikata H, Takahashi S, Takahashi I, Nishibuchi I, Doi Y, Kenjo M, Murakami Y, Honda Y, Kakizawa H et al (2015) Stereotactic body radiotherapy for patients with small hepatocellular carcinoma ineligible for resection or ablation therapies. Hepatol Res 45(4):378–386
Chiang CL, Chan MKH, Yeung CSY, Ho CHM, Lee FAS, Lee VWY, Wong FCS, Blanck O (2019) Combined stereotactic body radiotherapy and trans-arterial chemoembolization as initial treatment in BCLC stage B–C hepatocellular carcinoma. Strahlenther Onkol 195(3):254–264. https://doi.org/10.1007/s00066-018-1391-2
Jacob R, Turley F, Redden DT, Saddekni S, Aal AKA, Keene K, Yang E, Zarzour J, Bolus D, Smith JK et al (2015) Adjuvant stereotactic body radiotherapy following transarterial chemoembolization in patients with non-resectable hepatocellular carcinoma tumours of ≥ 3 cm. https://www.hpbonline.org/article/S1365-182X(15)31172-2/pdf. Accessed 27 July 2019
Shin HS, Hwan Lee S, Jun BG, Kim HS, Kang SH, Park JY, Choi SI, Cheon GJ, Kim YD, Yoo JJ et al (2022) Stereotactic body radiotherapy versus radiofrequency ablation as initial treatment of small hepatocellular carcinoma. Eur J Gastroenterol Hepatol 34(11):1187–1194
Wong TCL, Lee VHF, Law ALY, Pang HH, Lam KO, Lau V, Cui TY, Fong ASY, Lee SWM, Wong ECY et al (2021) Prospective study of stereotactic body radiation therapy for hepatocellular carcinoma on waitlist for liver transplant. Hepatology 74(5):2580
Lencioni R, Llovet JM Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. https://doi.org/10.1055/s-0030-1247132. Accessed 4 Jan 2020
Benson R, Madan R, Kilambi R, Chander S (2016) Radiation induced liver disease: a clinical update. J Egypt Natl Canc Inst 28(1):7–11
Chapman TR, Bowen SR, Schaub SK, Yeung RH, Kwan SW, Park JO, Yu L, Harris WP, Johnson GE, Liou IW et al (2018) Toward consensus reporting of radiation-induced liver toxicity in the treatment of primary liver malignancies: defining clinically relevant endpoints. Pract Radiat Oncol 8(3):157–166
Son SH, Jang HS, Lee H, Choi BO, Kang YN, Jang JW, Yoon SK, Kay CS (2013) Determination of the α/β ratio for the normal liver on the basis of radiation-induced hepatic toxicities in patients with hepatocellular carcinoma. Radiat Oncol 8(1):61
Lawremce TS, Dworzanin LM, Walker-Andrews SC, Andrews JC, Ten Haken RK, Wollmer IS, Lichter AS, Ensminger WD (1991) Treatment of cancers involving the liver and porta hepatis with external beam irradiation and intraarterial hepatic fluorodeoxyuridine. Int J Radiat Oncol Biol Phys 20(3):555–561
Durand-Labrunie J, Baumann A (2020) Curative irradiation treatment of hepatocellular carcinoma: a multicenter phase 2 trial. https://www.sciencedirect.com/science/article/pii/S0360301619345122. Accessed 3 Oct 2023
Il JW, Bae SH, Kim MS, Han CJ, Park SC, Kim SB, Cho EH, Choi CW, Kim KS, Hwang S et al (2020) A phase 2 multicenter study of stereotactic body radiotherapy for hepatocellular carcinoma: safety and efficacy. Cancer 126(2):363–372
Kimura T, Takeda A, Sanuki N, Ariyoshi K, Yamaguchi T, Imagumbai T, Katoh N, Eriguchi T, Oku Y, Ozawa S et al (2021) Multicenter prospective study of stereotactic body radiotherapy for previously untreated solitary primary hepatocellular carcinoma: the STRSPH study. Hepatol Res 51(4):461–471
Wahl DR, Stenmark MH, Tao Y, Pollom EL, Caoili EM, Lawrence TS, Schipper MJ, Feng M (2016) Outcomes after stereotactic body radiotherapy or radiofrequency ablation for hepatocellular carcinoma. J Clin Oncol 34(5):452–459. https://doi.org/10.1200/JCO.2015.61.4925
Kim N, Cheng J, Jung I, Liang JD, Shih YL, Huang WY, Kimura T, Lee VHF, Zeng ZC, Zhenggan R et al (2020) tereotactic body radiation therapy vs. radiofrequency ablation in Asian patients with hepatocellular carcinoma. J Hepatol 73(1):121–129
Sapir E, Tao Y, Schipper MJ, Bazzi L, Novelli PM, Devlin P, Owen D, Cuneo KC, Lawrence TS, Parikh ND et al (2018) Stereotactic body radiation therapy as an alternative to transarterial chemoembolization for hepatocellular carcinoma. Int J Radiat Oncol Biol Phys 100(1):122–130
Méndez Romero A, van der Holt B, Willemssen FEJA, de Man RA, Heijmen BJM, Habraken S, Westerveld H, van Delden OM, Klümpen HJ, Tjwa ETTL et al (2023) Transarterial chemoembolization with drug-eluting beads versus stereotactic body radiation therapy for hepatocellular carcinoma: outcomes from a multicenter, randomized, phase 2 trial (the TRENDY trial). Int J Radiat Oncol Biol Phys 117(1):45–52
Comito T, Loi M, Franzese C, Clerici E, Franceschini D, Badalamenti M, Teriaca MA, Rimassa L, Pedicini V, Poretti D et al (2022) Stereotactic radiotherapy after incomplete transarterial (chemo-) embolization (TAE\TACE) versus exclusive TAE or TACE for treatment of inoperable HCC: a phase III trial (NCT02323360). Curr Oncol 29(11):8802–8813
Brunner TB, Bettinger D, Schultheiss M, Maruschke L, Sturm L, Bartl N, Koundurdjieva I, Kirste S, Neeff HP, Goetz C et al (2021) Efficacy of stereotactic body radiotherapy in patients with hepatocellular carcinoma not suitable for transarterial chemoembolization (HERACLES: HEpatocellular Carcinoma Stereotactic RAdiotherapy CLinical efficacy study). Front Oncol. https://doi.org/10.3389/fonc.2021.653141
Ohri N, Tomé WA, Méndez Romero A, Miften M, Ten Haken RK, Dawson LA, Grimm J, Yorke E, Jackson A (2021) Local control after stereotactic body radiation therapy for liver tumors. Int J Radiat Oncol Biol Phys 110(1):188–195
Jang WI, Kim MS, Bae SH, Cho CK, Yoo HJ, Seo YS, Kang JK, Kim SY, Lee DH, Han CJ et al (2013) High-dose stereotactic body radiotherapy correlates increased local control and overall survival in patients with inoperable hepatocellular carcinoma. Radiat Oncol 8(1):1–12. https://doi.org/10.1186/1748-717X-8-250
Su TS, Liu QH, Zhu XF, Liang P, Liang SX, Lai L, Zhou Y, Huang Y, Cheng T, Li LQ (2021) Optimal stereotactic body radiotherapy dosage for hepatocellular carcinoma: a multicenter study. Radiat Oncol 16(1)
Dawson LA, Normolle D, Balter JM, Mcginn CJ, Lawrence TS, Ten Haken RK (2002) Analysis of radiation-induced liver disease using the Lyman NTCP model
Li JX, Zhang RJ, Qiu MQ, Yan LY, He ML, Long MY, Zhong JH, Lu HY, Zhou HM, Xiang B‑D et al (2023) Non-classic radiation-induced liver disease after intensity-modulated radiotherapy for Child-Pugh grade B patients with locally advanced hepatocellular carcinoma. Radiat Oncol 18(1):48
Bae S, Park H, Yoon W et al (2019) Treatment outcome after fractionated conformal radiotherapy for hepatocellular carcinoma in patients with Child-Pugh classification B in Korea (KROG 16-05). https://synapse.koreamed.org/articles/1153809. Accessed 6 Oct 2023
Sharma D, Thaper D, Kamal R, Yadav HP (2023) Role of palliative SBRT in barcelona clinic liver cancer-stage C hepatocellular carcinoma patients. Strahlenther Onkol 199(9):838–846. https://doi.org/10.1007/s00066-023-02065-x
Dawson LA, Winter KA, Knox JJ et al (2023) NRG/RTOG 1112: randomized phase III study of sorafenib vs. stereotactic body radiation therapy (SBRT) followed by sorafenib in hepatocellular carcinoma (HCC). J Clin Oncol 41(4):489. https://doi.org/10.1200/JCO.2023.41.4_suppl.489
Juloori A, Katipally RR, Lemons JM, Singh AK, Iyer R, Robbins JR, George B, Hall WA, Pitroda SP, Arif F et al (2023) Phase 1 randomized trial of stereotactic body radiation therapy followed by nivolumab plus ipilimumab or nivolumab alone in advanced/unresectable hepatocellular carcinoma. Int J Radiat Oncol Biol Phys 115(1):202–213
Gkika E, Strouthos I, Kirste S, Adebahr S, Schultheiss M, Bettinger D, Fritsch R, Brass V, Maruschke L, Neeff HP et al (2019) Repeated SBRT for in- and out-of-field recurrences in the liver. Strahlenther Onkol 195(3):246–253. https://doi.org/10.1007/s00066-018-1385-0
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
A.A. Mohamed, M.-L. Berres, P. Bruners, S.A. Lang, C. Trautwein, G. Wiltberger, A. Barabasch and M. Eble declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mohamed, A.A., Berres, ML., Bruners, P. et al. Managing hepatocellular carcinoma across the stages: efficacy and outcomes of stereotactic body radiotherapy. Strahlenther Onkol 200, 715–724 (2024). https://doi.org/10.1007/s00066-024-02235-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00066-024-02235-5