Abstract
Purpose
Dual-energy computed tomography (DECT) has been shown to be able to differentiate between intracranial hemorrhage (ICH) and extravasation of iodinated contrast media (contrast staining [CS]). TwinSpiral DECT is a recently introduced technique, which allows image acquisition at two different energy levels in two consecutive spiral scans. The aim of this study was to evaluate the feasibility and accuracy of TwinSpiral DECT to distinguish between ICH and CS after endovascular thrombectomy (EVT) in patients with acute ischemic stroke.
Methods
This retrospective single-center study conducted between November 2019 and July 2020 included non-contrast TwinSpiral DECT scans (tube voltages 80 and 150Sn kVp) of 39 ischemic stroke patients (18 females, 21 males, mean age 69 ± 11 years) within 48–72 h after endovascular thrombectomy. Parenchymal hyperdensity was assessed for the presence of ICH or/and CS by two board certified and fellowship-trained, blinded and independent neuroradiologists using standard mixed images and virtual non-contrast (VNC) images with corresponding iodine maps from TwinSpiral DECT. Follow-up examinations (FU; CT or MRI) were used as a standard of reference. Sensitivity, specificity, and accuracy for the detection of ICH as well as the inter-reader agreement were calculated.
Results
Parenchymal hyperdensities were detected in 17/39 (44%) patients. Using DECT, they were classified by both readers as ICH in 9 (53%), CS in 8 (47%), and mixture of both in 6 (35%) cases with excellent agreement (κ = 0.81, P < 0.0001). The sensitivity, specificity, and accuracy for the detection of ICH in DECT was 90% (95% confidence interval [CI]: 84–96%), 100% (95% CI 94–100%) and 95% (95% CI 89–100%), and in mixed images 90% (95% CI 84–96%), 86% (95% CI 80–92%) and 88% (95% CI 82–94%), respectively. Inter-reader agreement for detecting ICH on DECT compared to the mixed images was κ = 1.00 (P < 0.0001) vs. κ = 0.51 (P = 0.034).
Conclusion
TwinSpiral DECT demonstrates high accuracy and excellent specificity for differentiating ICH from CS in patients after mechanical thrombectomy due to acute ischemic stroke, and improves inter-reader agreement for detecting ICH compared to the standard mixed images.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Introduction
Endovascular thrombectomy (EVT) has become an indispensable treatment in selected patients with an acute ischemic stroke due to a large vessel occlusion.
Standard of care follow-up imaging is, among others, performed to rule out intracranial hemorrhage (ICH) and is recommended within 24 h after EVT, before administering anticoagulant or antiplatelet agents. Contrast staining (CS) is a frequently found phenomena after EVT in ischemic brain tissue due to a leakage of the blood brain barrier (contrast extravasation during the EVT). Unfortunately, CS might look very similar to the ICH in a conventional single-energy computed tomography (SECT) and might result in diagnostic uncertainty, misdiagnosis, and possibly the wrong treatment for the patient. Recent clinical studies have shown an importance of an early diagnosis of post-EVT hemorrhage, intraparenchymal or subarachnoid, and its impact on patient’s management and outcome [1, 2], thus more affordable and accessible techniques for improved hemorrhage detection are needed.
Dual-energy CT (DECT) is an advanced CT technique, which adds value based on the different x‑ray attenuation properties of tissue at high and low energy x‑rays. The two acquired datasets (low and high kV) from DECT enable material decomposition due to the unique high and low energy linear attenuation coefficients for a given material. Based on these characteristics, a three-material decomposition algorithm enables the differentiation between various materials with high atomic numbers, e.g. iodine, blood, or calcium [3,4,5,6].
Several clinical applications of DECT have been introduced in the field of neuroradiology [7], among them the generation of virtual non-contrast images and corresponding color encoded iodine maps. This approach allows removal of all iodine-containing structures from prior intravenous or intra-arterial iodine applications of an image (virtual non-contrast [VNC]) and to emphasize all iodine-containing structures (iodine maps). By using this method, previous studies have shown the ability of DECT to differentiate between ICH and CS after EVT [8, 9], as well as demonstrating the clinical value of this technique in imaging of patients with an acute ischemic stroke regarding decision making, outcome, prognosis and risk of ICH [1, 10].
TwinSpiral DECT, a version of the dual spiral technology, is a recently introduced DECT technique for acquiring datasets at two different energy levels for spectral separation. First, a low kV scan is acquired, immediately followed by a high kV tin filtered second scan, resembling a single scan by minimizing the delay between the two scans. To the best of our knowledge, no previous study investigated this technique for neuroradiological applications.
The aim of this study was to evaluate the feasibility and accuracy of TwinSpiral DECT to distinguish between hemorrhage and iodine extravasation after mechanical thrombectomy in patients with an acute ischemic stroke.
Material and Methods
Patient Selection
All procedures were performed in accordance with local and federal regulations and the Declaration of Helsinki. The retrospective study was approved by the local ethics committee (BASEC-Nr. 2018-01212).
In this retrospective cohort study, 39 patients (21 males, 18 females, mean age 69 ± 11 years, range 45–90 years) with a TwinSpiral DECT scan as follow-up after EVT in patients with acute ischemic stroke were included. Scans were performed between November 2019 and July 2020. Patients were identified through the institutional radiology information system with the search terms “dual-energy” and “mechanical thrombectomy” in the radiology reports. Inclusion criteria were the availability of TwinSpiral DECT images within 24 h after EVT and at least one additional follow-up examination within 48–72 h after the TwinSpiral CT scan, either done by a CT with a non-contrast phase, or a magnetic resonance imaging (MRI) with a susceptibility weighted imaging (SWI).
CT Data Acquisition
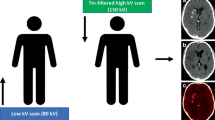
A head CT imaging was performed using a single-source CT scanner (Somatom X.cite, Siemens Healthcare, Erlangen, Germany) in TwinSpiral dual-energy mode. Tube voltages were set to 80 and 150 kVp, the latter operated with tin (Sn) filtration, and with corresponding quality reference tube current-time products of 220 and 179 mAs, respectively, using automated tube current modulation (CAREDose4D) (Fig. 1). Further scanning parameters were as follows: slice acquisition, 2 × 0.6 × 64 mm by means of a z-flying focal spot; rotation time, 1.0 s; and pitch, 0.55. The mean volume CT dose index (CTDIvol) of the protocol was 43.7 ± 3.4 mGy.
Schematic mode of operation of a single-source TwinSpiral dual-energy CT: Two consecutive scans at a different energies (low and a high kV scan) are performed directly one after the other. From these two datasets, conventional mixed CT images (a), as well as dedicated reconstructions, such as color-coded iodine overlay images (b) and virtual non-contrast (VNC) images (c) can be postprocessed
Postprocessing
All steps are part of the routine clinical protocol in DECT after mechanical thrombectomy at our department. Postprocessing and reconstruction was performed on a dedicated workstation (syngo MultiModality Workplace, CT Dual-Energy, Brain Hemorrhage, syngo.via, version VB.40 client 4.0, Siemens). The following postprocessing steps were semi-automatically performed by the scanner integrated software. First, conventional mixed CT images were reconstructed with a weighting factor of 0.5, representing a mix of images from the low-energy and high-energy scan (medium-smooth kernel, Hr40s) resembling single-energy CT [11, 12]. Second, color-coded iodine overlay images and VNC images were calculated from DECT TwinSpiral data by using the brain hemorrhage pre-set [4]. Image reconstruction of mixed, iodine maps, and VNC images was performed identically in an axial orientation with a slice thickness of 4 mm and increment of 4 mm. The underlying technical background for the differentiation of ICH and CS by DECT was described in detail elsewhere [13]. Briefly, an advanced three material decomposition algorithm was applied, which allows the differentiation of iodine, hemorrhage, and brain parenchyma.
After postprocessing all image data was transferred to our hospital’s picture archiving and communication system (Agfa, Version 6.6.1, Mortsel, Belgium).
Image Analysis
Two experienced and fellowship trained neuroradiologists performed the image analysis. The readout was carried out on high-resolution monitors (Flexscan MX 210, Eizo, Ishikawa, Japan) using the picture archiving and communication system (IMPAX EE R20, XVIII SU1, Agfa Healthcare, Bonn, Germany) of the hospital.
First, the image quality of standard mixed images and VNC and iodine maps reconstructions from TwinSpiral (VNC and iodine maps) was qualitatively rated as diagnostic or non-diagnostic regarding the evaluation of the brain parenchyma. Second, standard mixed and DECT images were evaluated regarding the presence of suspicious hyperdensities (yes/no, represents either CS or ICH) and further assessed based on the presence on iodine images (indicating CS) or on VNC images (representing ICH). In case of both hyperdensities in iodine maps and VNC images, a combination of CS and ICH was postulated. Third, with a time interval of 2 weeks, follow-up examinations (either non-contrast CT or MRI with SWI sequences were evaluated) as proof of evidence, as shown in previous studies [14]. CS in non-contrast follow-up CT was defined as a full or near complete washout of the hyperdensities detected in the initial CT scan after mechanical thrombectomy. ICH in a non-contrast follow-up CT was defined as a hyperdensity, which remained stable or near stable compared to the initial CT and if a typical ICH related perifocal edema was visible. ICH in MRI was defined as a susceptibility artifacts in SWI images [6, 15, 16]. Consensus reading was performed in case of discrepancies between readers. Both readers were blinded to the images or image reports of each of the previous or next steps of the image analysis and to the results of each other. After a period of more than 8 weeks, a re-readout of the scans by reader 1 for the purpose of intra-reader agreement was performed.
Statistical Analysis
Continuous variables were defined as mean ± standard deviation (SD) or median and range. Categorical variables were defined as frequencies and percentages.
Cohen κ coefficients were calculated to evaluate the inter-rater agreement regarding the classification of cases as ICH, CS or mixture of both using DECT images, as well as correctly detected ICH on DECT compared to the standard mixed images. κ values between 0.41 and 0.75 were interpreted as fair to good, and values between 0.75 and 1 were interpreted as excellent, according to criteria originally proposed by Landis and Koch [17].
Sensitivity, specificity, and accuracy for the correct assessment of the presence or absence of ICH were calculated. In cases of a combination of ICH and CS, sensitivity, specificity, and accuracy statistical calculations included these patients in the ICH group. Confidence intervals (CI) were computed at a level of 95%. Due to the small number of evaluated cases, exact confidence intervals based on binomial probabilities were calculated [18]. Follow-up images were used as a reference standard.
Results
A diagnostic image quality was found in all 39 (100%) patients for both mixed images and iodine and VNC reconstructions from TwinSpiral DECT in both readers (Figs. 2 and 3).
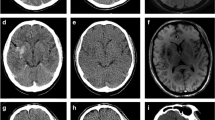
A standard native non-contrast computed tomography (CT) mixed image from TwinSpiral dual-energy CT shows a hyperdensity in the Sylvian cortex on the right side (blue arrow) in an acute ischemic stroke patient after endovascular thrombectomy (a). The hyperdensity is also seen in the same location on the virtual non-contrast (VNC) image (b). No hyperdensity is seen on the iodine map (c). Follow-up magnetic resonance image (MRI) (d) showing a susceptibility artifact (blue arrow) on a susceptibility-weighted imaging (SWI) corresponding to the hyperdense area seen on the standard mixed and VNC images, but not on the iodine map, thus proving an intracerebral hemorrhage
A hyperdensity is seen in the left basal ganglia (blue arrow) on a standard non-contrast mixed image from TwinSpiral dual-energy CT image in an acute ischemic stroke patient after endovascular thrombectomy (a). No hyperdensity is seen in the same location on the VNC image (b). Hyperdensity is seen on the iodine map (c). Follow-up non-contrast CT (d) showing no hyperdensity at the corresponding location (blue arrow), thus indicating the contrast extravasation resorbed in follow-up imaging
Hyperdensities were detected in 17 of the 39 cases (44%) in mixed and VNC images after EVT (κ = 1.00, P < 0.001). Using DECT (VNC and iodine maps), they were classified as ICH in 9 (53%), CS in 8 (47%), and a mixture of both in 6 (35%) cases with excellent agreement between readers (κ = 0.81, P < 0.0001).
For the TwinSpiral DECT the sensitivity, specificity, and accuracy for the correct detection of ICH using VNC and iodine maps were 90% (95% CI 84–96%), 100% (95% CI 94%–100%) and 95% (95% CI 89–100%), respectively. On the mixed images these same parameters resulted in respective values of 90% (95% CI 84–96%), 86% (95% CI 80–92%) and 88% (95% CI 82–94%) for both readers. Inter-reader agreement between the two readers for detecting ICH on DECT compared to the mixed images was κ = 1.00 (P < 0.0001) vs. κ = 0.51 (P = 0.034) (Fig. 4). Intra-reader agreement of reader 1 for detecting ICH on DECT and mixed images was κ = 1.00 and κ = 0.86 (P < 0.0001), respectively.
Discussion
This study introduces a novel dual-energy CT technique for the differentiation of CS from ICH after EVT in patients with acute stroke due to a large vessel occlusion. It indicates that the TwinSpiral DECT is feasible to separate these two entities and allows to clarify this clinically relevant question after mechanical thrombectomy. A high sensitivity, specificity, and accuracy for the presence of ICH in a non-contrast DECT suggests that this technique may be used in a clinical routine. The results of the study further indicate that the TwinSpiral DECT technique can be used as an alternative to the previously available DETC techniques, such as dual-source, rapid kilovoltage-switching, or dual-layer DECT.
To achieve different energies in a single acquisition, various DECT techniques have been introduced into a clinical imaging in recent years: a dual-source DECT with two x‑ray sources rotating simultaneously around the patient; a twin beam DECT with a filter-based splitting of the x‑ray beam, a rapid kilovoltage-switching DECT with a gemstone scintillator detector (GSI), a dual-layer detector-based (sandwich detector) spectral DECT technique and a photon-counting CT [19]. This requires dedicated CT scanners with either single or dual source, dual layer, or gemstone ability. The TwinSpiral technique, however, seems to be technically simpler. A tin filter provides a powerful spectral separation, while a stellar detector enables high-quality imaging at a temporal resolution in low-dose or low-signal imaging, thus providing low-dose DECT scans. Along with utilization of Recon&GO, this enables advanced comprehensive and automated routine-ready solution, well suited for departments with single-energetic scanners for less costs than dual-energetic scans, thus making it more accessible for a broader range of hospitals and providing potential cost-benefit.
A number of previous studies have shown benefits in utilization of DECT in stroke imaging protocols. This includes various aspects, such as differentiation of ICH and CS by using a dual-source DECT approach [9, 20], improved diagnosis and classification of ICH after EVT with a fast kVp-switching CT technique [1], hemorrhagic transformation after endovascular thrombectomy with potential to affect future antithrombotic strategy [21], quantitative assessment of the maximum iodine concentration within the suspicious hyperdensities [22], intracranial hemorrhage prediction [23], improved histological composition of a thrombus in an in vitro study [24] and a better visualization of early ischemic changes of brain tissue after mechanical thrombectomy [25], in addition to further applications in the field of neuroradiology, which include the differentiation between tumor bleeding and pure hemorrhage, or the automated bone removal in DECT angiography [8, 26,27,28]. At our department, the DECT is fully integrated in our routine clinical workflow after mechanical thrombectomy. The decision whether follow-up examinations require CT or MRI depends on the individual situation of each patient and on the scanner availability.
One of the drawbacks of the TwinSpiral technique could be its susceptibility regarding the patient motion. In case the patient moves between the low kV and the high kV scan, this potentially would result in a complete loss of the DECT information; however, the morphological information could still be retrieved e.g. from the low KV scan, even though a minor loss of quality can be expected here. In our series of TwinSpiral scans in acute stroke patients after mechanical thrombectomy, all scans demonstrated a diagnostic image quality. None of the patients demonstrated relevant motion between the low and the high kV scans.
We found two cases in where the parenchymal hyperdensities were classified as CS according to the DECT, but which demonstrated ICH in the follow-up MRI. In both patients, it was also retrospectively not possible to define with certainty whether bleeding was already present during the first follow-up examination, or whether it was a secondary hemorrhagic transformation or reperfusion hemorrhage, which occurred between the DECT and the follow-up imaging. Interestingly, in both cases the follow-up examination was made by MRI using SWI sequences, where tiny susceptibility artifacts were visible and therefore were interpreted as an ICH in our study. In such cases, it is difficult to accurately assess the diagnostic value of DECT due to the time difference in regard to the control imaging, in which additional changes may have occurred; however, it would be interesting to see how DECT can improve risk assessment of hemorrhagic transformation of ischemic territories, where CS is present.
We acknowledge the following study limitations. First, the number of patients included in this work was relatively low and additional studies with a higher number of cases are required to confirm our results. Second, the readers could not have been completely blinded, as the VNC and iodine map images could have been recognized due to their distinctive characteristics when evaluated by the DECT experienced readers. Third, we were not able to compare the TwinSpiral DECT to previous techniques, such as dual-source or rapid kilovoltage-switching. Finally, follow-up imaging was not performed at predefined time intervals and using the same imaging technique.
Conclusion
The VNC and iodine images derived from the TwinSpiral DECT enable an accurate detection of ICH after mechanical thrombectomy in patients with acute ischemic stroke. CS can be reliably differentiated from ICH which might have an impact on the prognosis, outcome and further therapeutic management of the patient.
References
Almqvist H, Holmin S, Mazya MV. Dual energy CT after stroke thrombectomy alters assessment of hemorrhagic complications. Neurology. 2019;93:e1068–75.
Renú A, Laredo C, Rodríguez-Vázquez A, Santana D, Werner M, Llull L, Lopez-Rueda A, Urra X, Rudilosso S, Obach V, Amaro S, Chamorro Á. Characterization of Subarachnoid Hyperdensities After Thrombectomy for Acute Stroke Using Dual-Energy CT. Neurology. 2022;98:e601–11.
Winklhofer S, Vittoria De Martini I, Nern C, Blume I, Wegener S, Pangalu A, Valavanis A, Alkadhi H, Guggenberger R. Dual-Energy Computed Tomography in Stroke Imaging: Technical and Clinical Considerations of Virtual Noncontrast Images for Detection of the Hyperdense Artery Sign. J Comput Assist Tomogr. 2017;41:843–8.
Johnson TR, Krauss B, Sedlmair M, Grasruck M, Bruder H, Morhard D, Fink C, Weckbach S, Lenhard M, Schmidt B, Flohr T, Reiser MF, Becker CR. Material differentiation by dual energy CT: initial experience. Eur Radiol. 2007;17:1510–7.
Siegel MJ, Kaza RK, Bolus DN, Boll DT, Rofsky NM, De Cecco CN, Foley WD, Morgan DE, Schoepf UJ, Sahani DV, Shuman WP, Vrtiska TJ, Yeh BM, Berland LL. White Paper of the Society of Computed Body Tomography and Magnetic Resonance on Dual-Energy CT, Part 1: Technology and Terminology. J Comput Assist Tomogr. 2016;40:841–5.
Mannil M, Winklhofer SF‑X. Neuroimaging techniques in clinical practice: physical concepts and clinical applications. Cham: Springer Nature; 2020.
Gibney B, Redmond CE, Byrne D, Mathur S, Murray N. A Review of the Applications of Dual-Energy CT in Acute Neuroimaging. Can Assoc Radiol J. 2020;71:253–65.
Phan CM, Yoo AJ, Hirsch JA, Nogueira RG, Gupta R. Differentiation of hemorrhage from iodinated contrast in different intracranial compartments using dual-energy head CT. AJNR Am J Neuroradiol. 2012;33:1088–94.
Tijssen MP, Hofman PA, Stadler AA, van Zwam W, de Graaf R, van Oostenbrugge RJ, Klotz E, Wildberger JE, Postma AA. The role of dual energy CT in differentiating between brain haemorrhage and contrast medium after mechanical revascularisation in acute ischaemic stroke. Eur Radiol. 2014;24:834–40.
Renú A, Amaro S, Laredo C, Román LS, Llull L, Lopez A, Urra X, Blasco J, Oleaga L, Chamorro Á. Relevance of blood-brain barrier disruption after endovascular treatment of ischemic stroke: dual-energy computed tomographic study. Stroke. 2015;46:673–9.
Yu L, Primak AN, Liu X, McCollough CH. Image quality optimization and evaluation of linearly mixed images in dual-source, dual-energy CT. Med Phys. 2009;36:1019–24.
Behrendt FF, Schmidt B, Plumhans C, Keil S, Woodruff SG, Ackermann D, Mühlenbruch G, Flohr T, Günther RW, Mahnken AH. Image fusion in dual energy computed tomography: effect on contrast enhancement, signal-to-noise ratio and image quality in computed tomography angiography. Invest Radiol. 2009;44:1–6.
Hu R, Padole A, Gupta R. Dual-Energy Computed Tomographic Applications for Differentiation of Intracranial Hemorrhage, Calcium, and Iodine. Neuroimaging Clin N Am. 2017;27:401–9.
Chen W, Zhu W, Kovanlikaya I, Kovanlikaya A, Liu T, Wang S, Salustri C, Wang Y. Intracranial calcifications and hemorrhages: characterization with quantitative susceptibility mapping. Radiology. 2014;270:496–505.
Yoon W, Seo JJ, Kim JK, Cho KH, Park JG, Kang HK. Contrast enhancement and contrast extravasation on computed tomography after intra-arterial thrombolysis in patients with acute ischemic stroke. Stroke. 2004;35:876–81.
Nakano S, Iseda T, Yoneyama T, Wakisaka S. Early CT signs in patients with acute middle cerebral artery occlusion: incidence of contrast staining and haemorrhagic transformations after intra-arterial reperfusion therapy. Clin Radiol. 2006;61:156–62.
Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–74.
Deeks JJ, Altman DG. Sensitivity and specificity and their confidence intervals cannot exceed 100%. BMJ. 1999;318:193–4.
Forghani R, De Man B, Gupta R. Dual-Energy Computed Tomography: Physical Principles, Approaches to Scanning, Usage, and Implementation: Part 1. Neuroimaging Clin N Am. 2017;27:371–84.
Gupta R, Phan CM, Leidecker C, Brady TJ, Hirsch JA, Nogueira RG, Yoo AJ. Evaluation of dual-energy CT for differentiating intracerebral hemorrhage from iodinated contrast material staining. Radiology. 2010;257:205–11.
Liu K, Jiang L, Ruan J, Xia W, Huang H, Niu G, Yan S, Yin C. The Role of Dual Energy CT in Evaluating Hemorrhagic Complications at Different Stages After Thrombectomy. Front Neurol. 2020;11:583411.
Bonatti M, Lombardo F, Zamboni GA, Vittadello F, Currò Dossi R, Bonetti B, Pozzi Mucelli R, Bonatti G. Iodine Extravasation Quantification on Dual-Energy CT of the Brain Performed after Mechanical Thrombectomy for Acute Ischemic Stroke Can Predict Hemorrhagic Complications. AJNR Am J Neuroradiol. 2018;39:441–7.
Byrne D, Walsh JP, Schmiedeskamp H, Settecase F, Heran MKS, Niu B, Salmeen AK, Rohr B, Field TS, Murray N, Rohr A. Prediction of Hemorrhage after Successful Recanalization in Patients with Acute Ischemic Stroke: Improved Risk Stratification Using Dual-Energy CT Parenchymal Iodine Concentration Ratio Relative to the Superior Sagittal Sinus. AJNR Am J Neuroradiol. 2020;41:64–70.
Borggrefe J, Kottlors J, Mirza M, Neuhaus VF, Abdullayev N, Maus V, Kabbasch C, Maintz D, Mpotsaris A. Differentiation of Clot Composition Using Conventional and Dual-Energy Computed Tomography. Clin Neuroradiol. 2018;28:515–22.
Grams AE, Djurdjevic T, Rehwald R, Schiestl T, Dazinger F, Steiger R, Knoflach M, Gizewski ER, Glodny B. Improved visualisation of early cerebral infarctions after endovascular stroke therapy using dual-energy computed tomography oedema maps. Eur Radiol. 2018;28:4534–41.
Kim SJ, Lim HK, Lee HY, Choi CG, Lee DH, Suh DC, Kim SM, Kim JK, Krauss B. Dual-energy CT in the evaluation of intracerebral hemorrhage of unknown origin: differentiation between tumor bleeding and pure hemorrhage. AJNR Am J Neuroradiol. 2012;33:865–72.
Choi Y, Shin NY, Jang J, Ahn KJ, Kim BS. Dual-energy CT for differentiating acute intracranial hemorrhage from contrast staining or calcification: a meta-analysis. Neuroradiology. 2020;62:1617–26.
Brockmann C, Scharf J, Nölte IS, Seiz M, Groden C, Brockmann MA. Dual-energy CT after peri-interventional subarachnoid haemorrhage: a feasibility study. Clin Neuroradiol. 2010;20:231–5.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Funding
Open access funding provided by University of Zurich
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
R. Grkovski, L. Acu, U. Ahmadli, R. Terziev, T. Schubert, S. Wegener, Z. Kulcsár, S. Husain, H. Alkadhi and S. Winklhofer declare that they have no competing interests.
Ethical standards
Ethical approval: This study was approved by the local ethics committee of the University Hospital Zürich (BASEC-Nr. 2018-01212). The study was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. Consent to participate: all participants of the study gave their written informed consent prior to examination. Consent for publication: all participants gave approval for the data to be published.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Grkovski, R., Acu, L., Ahmadli, U. et al. A Novel Dual-Energy CT Method for Detection and Differentiation of Intracerebral Hemorrhage From Contrast Extravasation in Stroke Patients After Endovascular Thrombectomy. Clin Neuroradiol 33, 171–177 (2023). https://doi.org/10.1007/s00062-022-01198-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00062-022-01198-3