Abstract
Objective
To assess if a new dual-energy computed tomography (DECT) technique enables an improved visualization of ischemic brain tissue after mechanical thrombectomy in acute stroke patients.
Material and Methods
The DECT head scans with a new sequential technique (TwinSpiral DECT) were performed in 41 patients with ischemic stroke after endovascular thrombectomy and were retrospectively included. Standard mixed and virtual non-contrast (VNC) images were reconstructed. Infarct visibility and image noise were assessed qualitatively by two readers using a 4-point Likert scale. Quantitative Hounsfield units (HU) were used to assess density differences of ischemic brain tissue versus healthy tissue on the non-affected contralateral hemisphere.
Results
Infarct visibility was significantly better in VNC compared to mixed images for both readers R1 (VNC: median 1 (range 1–3), mixed: median 2 (range 1–4), p < 0.05) and R2 (VNC: median 2 (range 1–3), mixed: 2 (range 1–4), p < 0.05). Qualitative image noise was significantly higher in VNC compared to mixed images for both readers R1 (VNC: median 3, mixed: 2) and R2 (VNC: median 2, mixed: 1, p < 0.05, each). Mean HU were significantly different between the infarcted tissue and the reference healthy brain tissue on the contralateral hemisphere in VNC (infarct 24 ± 3) and mixed images (infarct 33 ± 5, p < 0.05, each). The mean HU difference between ischemia and reference in VNC images (mean 8 ± 3) was significantly higher (p < 0.05) compared to the mean HU difference in mixed images (mean 5 ± 4).
Conclusion
TwinSpiral DECT allows an improved qualitative and quantitative visualization of ischemic brain tissue in ischemic stroke patients after endovascular treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Stroke is the second leading cause of mortality worldwide and an important contributor to disability [1]. Numerous studies have shown the benefit of endovascular mechanical thrombectomy (EVT) in patients with large vessel stroke [2, 3]. Follow-up imaging after the intervention can be performed to assess the extension of the ischemic brain tissue, to rule out intracranial hemorrhage, to evaluate the vessel status and to exclude further potential complications. As this might impact the ongoing treatment as well as the prognosis for the patient, an affordable and easily accessible one stop imaging technique is one of the focuses in stroke imaging and assessment. Dual-energy computed tomography (DECT) can be a helpful diagnostic imaging modality in order to optimize these imaging tasks. Currently there are a number of available DECT techniques of the main vendors, such as: (1) dual-source X‑ray tubes performing simultaneous acquisitions using different tube voltages (e.g., 80–100 kV and 140–150 kV), single-source rapid voltage switching between two tube voltages, a single-source split beam into two different energy spectra and a dual-layer detector with simultaneous data acquisition of the low-energy and high-energy dataset [4,5,6,7]. Various DECT applications have been implemented in stroke assessment, for example, in differentiating acute intracranial hemorrhage from contrast staining or calcification [8, 9], visualization of ischemic changes and brain edema [5, 10, 11], prediction of infarction development or hemorrhagic transformation in contrast-enhancing areas [12], and detecting a hyperdense artery sign [13]. Recently, a new technique has been developed, TwinSpiral DECT, a variant of dual spiral DECT, which uses a tin filter and an improved detector enabling low-dose dual-energy scans for optimal spectral separation with a better soft tissue contrast [14]. It acquires two sequential scans in a very short time, one with high and one with low energy immediately after each other.
A recent publication has shown the feasibility of TwinSpiral DECT to differentiate between intracranial hemorrhage and iodine staining with a high accuracy [15]. The aim of the current study was to assess whether TwinSpiral DECT allows an improved visualization of ischemic brain tissue after mechanical thrombectomy.
Material and Methods
Patients
All procedures were performed in accordance with local and federal regulations and the Declaration of Helsinki. The study was approved by the local ethics committee (approval number 2018-01212). All patients with an acute ischemic stroke due to middle cerebral artery occlusion (M1 or proximal M2 segment) and consequential mechanical thrombectomy between September 2019 and December 2020 were retrospectively reviewed. Inclusion criteria were a patient age > 18 years and the availability of a TwinSpiral DECT scan within 24 h after thrombectomy and exclusion criteria were an extensive intracranial hemorrhage or materials producing substantial artifacts. A total of 41 patients (18 women, 44%, 23 men, 56%, mean age 73 ± 11.2 years, range 50–93 years) were included in the study analysis.
Imaging and Postprocessing
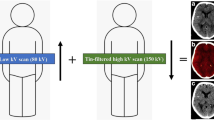
An unenhanced DECT scan was performed in all patients using a single source Twin-Spiral DECT scanner (X.cite, Siemens Healthineers, Forchheim, Germany). Postinterventional follow-up imaging after mechanical thrombectomy is part of a standard routine process for patients after acute ischemic stroke at the Department of Neuroradiology, University Hospital Zurich. For image acquisition, the following parameters were used: Tube voltages were set to 80 kV and tin (Sn) filtered 150 kV (effective reference of 379mA and 318mA, respectively) (Fig. 1). A slice thickness of 1.5 mm, a pitch factor of 0.55 and an incremental acquisition mode with a 64 × 0.6 mm collimation were used. The mean computed tomography dose index volume (CTDIvol) was 43.6 ± 3.7 mGy. Image reconstruction included both virtual non-contrast (VNC) and standard mixed images in axial orientation and an image matrix of 512 × 512 mm, with a slice thickness of 4 mm and an increment of 4 mm. A convolution Kernel Qr40 was used for VNC and a Hr40 for standard mixed images. Standard mixed images, which are used for routine clinical interpretation are weighted images generated from the two energies (80 and 150 kV) resembling a conventional polychromatic single-energy head CT scan with 120 kVp [16,17,18]. These reconstructions were performed at a dedicated postprocessing workstation (syngo MultiModality Workplace, CT Dual-Energy, Virtual-Unenhanced application, syngo.via, version VB.40 client 4.0, Siemens Healthineers AG, Erlangen, Germany).
Schematic representation of a single-source TwinSpiral DECT scan and reconstructions. Two consecutive scans at a different energy (low and a high kV scan) are performed consecutively. From these two datasets, conventional mixed CT images (a), virtual non-contrast (VNC) images (b), and color-coded iodine overlay images (c) can be postprocessed
Image Analysis
Image analysis included a quantitative and qualitative image readout of the standard mixed and VNC images. The readout was performed on high-resolution monitors (Flexscan MX 210, Eizo, Ishikawa, Japan), using the picture archiving and communication system (IMPAX EE R20, XVIII SU1, Agfa Healthcare, Bonn, Germany) of the hospital.
Qualitative Image Analysis
Qualitative image analysis was performed independently by two experienced board certified and fellowship-trained and independent neuroradiologists (R1 with 9 years of experience in radiology and 6 in neuroradiology, and R2 with 16 years of experience in radiology and 4 in neuroradiology). Both readers were blinded to the image type of reconstruction (VNC or mixed images), clinical patient information and to the results from the second reader. Image windowing was performed individually by each reader. Images were presented to the readers by a third person, who presented patients and their image reconstructions in a random and arbitrary order to both readers.
First, the image quality was evaluated as diagnostic or non-diagnostic. Non-diagnostic criteria included images with severe impairment due to motion or metal artifacts. Second, ischemic changes of the brain parenchyma were assessed by determining the visibility of the contrast between affected hypodense ischemic tissue and unaffected, healthy tissue (1 = good, 2 = moderate, 3 = little contrast, 4 = no contrast). Third, image noise was evaluated in the same regions as previous contrast assessment by grading it into 4 categories (1 = no noise, 2 = little noise with no disturbance of the infarction evaluation, 3 = moderate noise with little disturbance of the infarction evaluation, 4 = severe noise with marked disturbance of the infarction evaluation).
Quantitative Image Analysis
Quantitative image analysis was performed by a board certified, fellowship trained neuroradiologist (UA, with 4 years of experience in neuroradiology). The reader opened both standard mixed and VNC images. A region of interest (ROI) was placed in the affected area and was mirrored in the unaffected area on the contralateral healthy hemisphere for each image reconstruction separately. The ROI size was at least 1 cm2. Average Hounsfield units (HU) and the standard deviation (SD) were noted for each ROI in both VNC and standard mixed images.
Statistical Analysis
Descriptive statistics are provided as mean ± standard deviation (range) for continuous and frequencies (n) and as median and range values for categorical variables. The Shapiro-Wilk test was performed to investigate for normality distribution.
Cohen’s κ‑coefficients were calculated to evaluate the inter-rater agreement regarding the assessment of contrast and image noise in VNC and mixed images. The κ values below 0.4 were interpreted as poor and between 0.41–0.75 as fair to good according to criteria originally proposed by Landis and Koch [19].
Comparison of qualitative scores (contrast and image noise) between VNC and mixed images was conducted using the pairwise Wilcoxon signed rank test to assess for significant differences.
For quantitative analysis, a statistical significance of mean in differences of HU values for each reconstruction modality was determine using the paired Student’s t‑test. A p-value < 0.05 was considered statistically significant. Statistical analyses were conducted using commercially available software (IBM SPSS Statistics, Version 26.0, IBM Corp. Armonk, NY, USA and Microsoft Excel, Microsoft Corporation. (2019). Microsoft Excel. Retrieved from https://office.microsoft.com/excel).
Results
Inter-Rater Agreement
The inter-rater agreement regarding the contrast and qualitative image noise between R1 and R2 was low (k < 0.4; p < 0.0001); hence qualitative statistical results are presented for each reader separately.
Qualitative Results
Both readers rated the overall image quality as diagnostic in all cases and reconstructions. The contrast score was significantly better in VNC images compared to mixed images for both readers R1 (VNC: median 1 (range 1–3), mixed: 2 (range 1–4), p < 0.05) and R2 (VNC: median 2 (range 1–3), mixed: 2 (range 1–4), p < 0.05) (Figs. 2, 3 and 4).
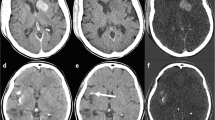
A patient with a right Sylvian artery ischemic stroke. a Standard mixed images and b virtual non-contrast images (VNC) reconstructed from a TwinSpiral DECT acquisition. Ischemic hypodense area of infarction is better visible on the VNC (yellow arrow) compared to the standard mixed images. It shows better contrast and larger impacted area
a Standard mixed images and b virtual non-contrast images (VNC). Note the old right hemispheric infarction is clearly visible in both standard mixed and VNC images (blue arrow). However, the acute hypodense ischemic area on the contralateral left hemisphere is much better differentiated on the VNC images (yellow arrows) compared to the standard mixed images
The qualitative image noise was significantly higher in VNC images compared to mixed images for both readers R1 (VNC: median 3 (range 2–3); mixed: 2 (range 2–3), p < 0.05) and R2 (VNC: median 2 (range 1–3); mixed: 1 (range 1–3), p < 0.05) (Fig. 4).
Quantitative Image Analysis
Mean HU were significantly different between the infarcted tissue and the reference healthy brain tissue on the contralateral hemisphere in VNC (infarct 24 ± 3 HU, reference 31 ± 2 HU) and mixed images (infarct 33 ± 5 HU, reference 38 ± 3 HU) (p < 0.05, each). The mean HU difference between VNC ischemia and VNC reference (mean 8 ± 3 HU) was significantly higher (p < 0.05) compared to the mean HU difference in mixed images in ischemia and reference tissue (mean 5 ± 4) (Figs. 5 and 6).
A patient with a right Sylvian artery acute ischemic stroke. Hounsfield units (HU) measurements on the right (affected) and contralateral left (unaffected) side. a Standard mixed images: region of interest (ROI) 1—39 HU, ROI 2—42 HU, b virtual non-contrast images (VNC): ROI 3—25 HU, ROI 4—33 HU. After the subtraction of values for each image modality, the difference in the VNC images (factor of 8) is bigger compared to the standard mixed images (factor of 3)
Discussion
This study investigated the value and feasibility of a new DECT technique: TwinSpiral DECT, for an improved ischemic stroke detection and visualization. Our results suggest that TwinSpiral DECT is a feasible method to be performed in patients after mechanical thrombectomy with an improved visualization of the ischemic tissue in VNC compared to the standard mixed images; however, despite our paper focusing on patients post-EVT, as DECT is primarily performed in patients after EVT, the better contrast visibility between healthy and ischemic tissue could be implied in initial stroke assessment as well. Thus, the next step would be a study in which this DECT technique would be applied for the initial imaging in the acute ischemic stroke setting (i.e. before EVT) to assess the benefit of VNC images for the detection of ischemic brain tissue and therefore for a potentially more accurate clinical prognosis and optimal therapeutic management.
By comparing the qualitative values of each individual reader, the VNC images from the tested DECT demonstrated the affected ischemic brain tissue to be significantly more hypodense than in standard mixed images, although with a slight increase of noise. The infarcted areas were better visible in VNC images compared to standard mixed images. Measurements of HU and comparing the values of VNC and standard mixed images showed a markedly increased difference in radiodensity between ischemic and healthy brain tissue, thus allowing an improved delineation and assessment of ischemic stroke.
Detecting ischemic changes post-EVT is just as important as pre-EVT. The distant embolization of the affected vessel and infarcts in new vascular territories are both associated with worse prognosis due to newly developed ischemic regions [20, 21] and impairment of collateral blood flow to already affected areas [22]. Prompt treatment, either by EVT or intra-arterial thrombolysis, is important for favorable clinical outcome and prevention of further complications [23].
The results of our study are comparable to results from Gariani et al. [10]. They have shown to be able to separate ischemic from non-ischemic tissue with major advantages in DECT by using VNC compared to non-contrast CT weighted sum images (NCCT). Another study showed superiority of VNC images compared to conventional images (using a dual-layer spectral CT) in ischemic infarct delineation [24], as well as in detection of ischemic changes in posterior fossa [25]; however, both studies were performed on a DECT acquisition technology other than TwinSpiral DECT. In addition, previous studies have also reported a higher noise in both VNC [26] and virtual monoenergetic imaging [27, 28], which was also observed in our study. Nevertheless, the increased image noise seems not to have a major impact on the detection of hypodense ischemic brain tissue.
The first concept of DECT was mentioned by Hounsfield in 1973, when he described acquiring two separate images (at 100 kV and the other at 140 kV) at the same slice, in order to differentiate iodine from calcium based on different atomic numbers [29]. This required two scans for a single series of images, thus approximately doubling the radiation dose. Recently, a new DECT image technique was developed, TwinSpiral DECT, enabling two scans at low-kV and high-kV data sets integrated into one single acquisition, without an increase in radiation dose. This method does not require dual source, dual layer, or rapid energy switching ability. Instead, a tin filter provides powerful spectral separation [30, 31], while an improved detector used herein enables high-quality imaging at a high temporal resolution in low dose or low signal imaging [14], with utilization of advanced (semi-)automated postprocessing algorithms enabling facilitated routine-ready solutions. As such, it may be applied by a broader pool of health institutions, with less costs than other high-end DECT scanners.
One of the drawbacks of the TwinSpiral DECT technique could be its susceptibility to motion artefacts, such as in agitated patients, where the movement of the patient between the low kV and the high kV scan could potentially compromise the quality of DECT images. In our series, all of the included studies demonstrated diagnostic image quality. No major motion artifacts were seen, which also reflects our personal clinical experience from daily routine with this new dual energy technique; however, even in the case of patient movement in one of the two scans, the second scan could still be used for diagnostic purposes (even though the dual-energy information might get lost). Furthermore, it is important to note that the mean radiation dose was within the range of a normal single-energy head CT scan and in the range of the diagnostic reference levels (DRLs) and achievable doses (ADs) in the USA [32].
In addition to the above investigated application, the TwinSpiral DECT technique might also provide further already established benefits of a DECT, not only in detecting ischemic changes, but differentiating between hemorrhage [15], iodine, calcifications and metal artifacts, without the need for multiple scans [33, 34]. Furthermore, this study might open new frontiers in stroke research, as to evaluate the technique’s ability for ischemic stroke in the posterior circulation or for an improved stroke detection in initial patient presentation.
Although there was somewhat of a discrepancy between inter-reader agreement in qualitative image analysis in our study, each reader represented a separate control, and what is most important, both readers were able to detect significant changes in contrast between VNC and standard mixed images, a key finding in ischemic brain tissue.
Limitations of our study were the relatively small retrospective study size and blinding, which was not completely possible, as experienced readers could easily recognize the type of DECT imaging reconstruction. Furthermore, qualitative image analysis could be done by ASPECTS, which, in future studies including more patients, might be used to assess prognosis in pre-EVT in post-EVT patients.
Conclusion
Our study demonstrated that TwinSpiral as a new DECT technique allows an improved qualitative and quantitative visualization of ischemic brain tissue. This indicates that TwinSpiral DECT might be a valuable tool for imaging of ischemic stroke patients after endovascular treatment.
References
Katan M, Luft A. Global burden of stroke. Semin Neurol. 2018;38:208–11. https://doi.org/10.1055/s-0038-1649503.
Papanagiotou P, Ntaios G. Endovascular thrombectomy in acute Ischemic stroke. Circ Cardiovasc Interv. 2018;11:e5362. https://doi.org/10.1161/CIRCINTERVENTIONS.117.005362. in eng.
Casetta I, Fainardi E, Saia V, Pracucci G, Padroni M, Renieri L, Nencini P, Inzitari D, Morosetti D, Sallustio F, Vallone S, Bigliardi G, Zini A, Longo M, Francalanza I, Bracco S, Vallone IM, Tassi R, Bergui M, Naldi A, Saletti A, De Vito A, Gasparotti R, Magoni M, Castellan L, Serrati C, Menozzi R, Scoditti U, Causin F, Pieroni A, Puglielli E, Casalena A, Sanna A, Ruggiero M, Cordici F, Di Maggio L, Duc E, Cosottini M, Giannini N, Sanfilippo G, Zappoli F, Cavallini A, Cavasin N, Critelli A, Ciceri E, Plebani M, Cappellari M, Chiumarulo L, Petruzzellis M, Terrana A, Cariddi LP, Burdi N, Tinelli A, Auteri W, Silvagni U, Biraschi F, Nicolini E, Padolecchia R, Tassinari T, Filauri P, Sacco S, Pavia M, Invernizzi P, Nuzzi NP, Marcheselli S, Amistà P, Russo M, Gallesio I, Craparo G, Mannino M, Mangiafico S, Toni D; Italian Registry of Endovascular Treatment in Acute Stroke. Endovascular Thrombectomy for Acute Ischemic Stroke Beyond 6 Hours From Onset: A Real-World Experience. Stroke. 2020;51:2051–7. https://doi.org/10.1161/STROKEAHA.119.027974.
Goo HW, Goo JM. Dual-energy CT: new horizon in medical imaging. Korean J Radiol. 2017;18:555–69. https://doi.org/10.3348/kjr.2017.18.4.555.
Mangesius S, Janjic T, Steiger R, Haider L, Rehwald R, Knoflach M, Widmann G, Gizewski E, Grams A. Dual-energy computed tomography in acute ischemic stroke: state-of-the-art. Eur Radiol. 2021;31:4138–47. https://doi.org/10.1007/s00330-020-07543-9.
Almeida IP, Schyns LE, Öllers MC, van Elmpt W, Parodi K, Landry G, Verhaegen F. Dual-energy CT quantitative imaging: a comparison study between twin-beam and dual-source CT scanners. Med Phys. 2017;44:171–9. https://doi.org/10.1002/mp.12000.
De Santis D, et al. Heavily calcified coronary arteries: advanced calcium subtraction improves luminal visualization and diagnostic confidence in dual-energy coronary computed Tomography Angiography. Invest Radiol. 2018;53:103–9. https://doi.org/10.1097/RLI.0000000000000416.
Yedavalli V, Sammet S. Contrast extravasation versus hemorrhage after thrombectomy in patients with acute stroke. J Neuroimaging. 2017;27:570–6. https://doi.org/10.1111/jon.12446.
Choi Y, Shin NY, Jang J, Ahn KJ, Kim BS. Dual-energy CT for differentiating acute intracranial hemorrhage from contrast staining or calcification: a meta-analysis. Neuroradiology. 2020;62:1617–26. https://doi.org/10.1007/s00234-020-02486-w.
Gariani J, Cuvinciuc V, Courvoisier D, Krauss B, Mendes Pereira V, Sztajzel R, Lovblad KO, Vargas MI. Diagnosis of acute ischemia using dual energy CT after mechanical thrombectomy. J Neurointerv Surg. 2016;8:996–1000. https://doi.org/10.1136/neurintsurg-2015-011988.
Mohammed MF, Marais O, Min A, Ferguson D, Jalal S, Khosa F, OʼKeeffe M, OʼConnell T, Schmiedeskamp H, Krauss B, Rohr A, Nicolaou S. Unenhanced Dual-Energy Computed Tomography: Visualization of Brain Edema. Invest Radiol. 2018;53:63–9. https://doi.org/10.1097/RLI.0000000000000413.
Djurdjevic T, Rehwald R, Knoflach M, Matosevic B, Kiechl S, Gizewski ER, Glodny B, Grams AE. Prediction of infarction development after endovascular stroke therapy with dual-energy computed tomography. Eur Radiol. 2017;27:907–17. https://doi.org/10.1007/s00330-016-4412-5.
Winklhofer S, Vittoria De Martini I, Nern C, Blume I, Wegener S, Pangalu A, Valavanis A, Alkadhi H, Guggenberger R. Dual-Energy Computed Tomography in Stroke Imaging: Technical and Clinical Considerations of Virtual Noncontrast Images for Detection of the Hyperdense Artery Sign. J Comput Assist Tomogr. 2017;41:843–8. https://doi.org/10.1097/RCT.0000000000000638.
Chaikriangkrai K, Choi SY, Nabi F, Chang SM. Important advances in technology and unique applications to cardiovascular computed tomography. Methodist Debakey Cardiovasc J. 2014;10:152–8. https://doi.org/10.14797/mdcj-10-3-152.
Grkovski R, Acu L, Ahmadli U, Terziev R, Schubert T, Wegener S, Kulcsar Z, Husain S, Alkadhi H, Winklhofer S. A Novel Dual-Energy CT Method for Detection and Differentiation of Intracerebral Hemorrhage From Contrast Extravasation in Stroke Patients After Endovascular Thrombectomy : Feasibility and First Results. Clin Neuroradiol. 2022; https://doi.org/10.1007/s00062-022-01198-3. Epub ahead of print.
Winklhofer S, Benninger E, Spross C, Morsbach F, Rahm S, Ross S, Jost B, Thali MJ, Stolzmann P, Alkadhi H, Guggenberger R. CT metal artefact reduction for internal fixation of the proximal humerus: value of mono-energetic extrapolation from dual-energy and iterative reconstructions. Clin Radiol. 2014;69:e199–206. https://doi.org/10.1016/j.crad.2013.12.011.
Naruto N, Itoh T, Noguchi K. Dual energy computed tomography for the head. Jpn J Radiol. 2018;36:69–80. https://doi.org/10.1007/s11604-017-0701-4.
Forghani R, De Man B, Gupta R. Dual-energy computed tomography: physical principles, approaches to scanning, usage, and implementation: part 2. Neuroimaging Clin N Am. 2017;27:385–400. https://doi.org/10.1016/j.nic.2017.03.003.
Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–74.
Ganesh A, Al-Ajlan FS, Sabiq F, Assis Z, Rempel JL, Butcher K, Thornton J, Kelly P, Roy D, Poppe AY, Jovin TG, Devlin T, Baxter BW, Krings T, Casaubon LK, Frei DF, Choe H, Tampieri D, Teitelbaum J, Lum C, Mandzia J, Phillips SJ, Bang OY, Almekhlafi MA, Coutts SB, Barber PA, Sajobi T, Demchuk AM, Eesa M, Hill MD, Goyal M, Menon BK; ESCAPE Trial Investigators. Infarct in a New Territory After Treatment Administration in the ESCAPE Randomized Controlled Trial (Endovascular Treatment for Small Core and Anterior Circulation Proximal Occlusion With Emphasis on Minimizing CT to Recanalization Times). Stroke. 2016;47:2993–8. https://doi.org/10.1161/STROKEAHA.116.014852.
Gascou G, Lobotesis K, Machi P, Maldonado I, Vendrell JF, Riquelme C, Eker O, Mercier G, Mourand I, Arquizan C, Bonafé A, Costalat V. Stent retrievers in acute ischemic stroke: complications and failures during the perioperative period. AJNR Am J Neuroradiol. 2014;35:734–40. https://doi.org/10.3174/ajnr.A3746.
Gratz PP, Schroth G, Gralla J, Mattle HP, Fischer U, Jung S, Mordasini P, Hsieh K, Verma RK, Weisstanner C, El-Koussy M. Whole-Brain Susceptibility-Weighted Thrombus Imaging in Stroke: Fragmented Thrombi Predict Worse Outcome. AJNR Am J Neuroradiol. 2015;36:1277–82. https://doi.org/10.3174/ajnr.A4275.
Pilgram-Pastor SM, Piechowiak EI, Dobrocky T, Kaesmacher J, Den Hollander J, Gralla J, Mordasini P. Stroke thrombectomy complication management. J Neurointerv Surg. 2021;13:912–7. https://doi.org/10.1136/neurintsurg-2021-017349.
Riederer I, Fingerle AA, Baum T, Kirschke JS, Rummeny EJ, Noël PB, Pfeiffer D. Acute infarction after mechanical thrombectomy is better delineable in virtual non-contrast compared to conventional images using a dual-layer spectral CT. Sci Rep. 2018;8:9329. https://doi.org/10.1038/s41598-018-27437-7.
Hixson HR, Leiva-Salinas C, Sumer S, Patrie J, Xin W, Wintermark M. Utilizing dual energy CT to improve CT diagnosis of posterior fossa ischemia. J Neuroradiol. 2016;43:346–52. https://doi.org/10.1016/j.neurad.2016.04.001.
Lehti L, Söderberg M, Höglund P, Nyman U, Gottsäter A, Wassélius J. Reliability of virtual non-contrast computed tomography angiography: comparing it with the real deal. Acta Radiol Open. 2018;7:2058460118790115. https://doi.org/10.1177/2058460118790115.
D’Angelo T, Cicero G, Mazziotti S, Ascenti G, Albrecht MH, Martin SS, Othman AE, Vogl TJ, Wichmann JL. Dual energy computed tomography virtual monoenergetic imaging: technique and clinical applications. Br J Radiol. 2019;92:20180546. https://doi.org/10.1259/bjr.20180546.
Liu CK, Huang HM. Noise reduction in dual-energy computed tomography virtual monoenergetic imaging. J Appl Clin Med Phys. 2019;20:104–13. https://doi.org/10.1002/acm2.12694.
Hounsfield GN. Computerized transverse axial scanning (tomography): Part I. Description of system. 1973. Br J Radiol. 1995;68:H166–72.
Kaufmann S, Sauter A, Spira D, Gatidis S, Ketelsen D, Heuschmid M, Claussen CD, Thomas C. Tin-filter enhanced dual-energy-CT: image quality and accuracy of CT numbers in virtual noncontrast imaging. Acad Radiol. 2013;20:596–603. https://doi.org/10.1016/j.acra.2013.01.010.
Choi SJ, Ahn SJ, Park SH, Park SH, Pak SY, Choi JW, Shim YS, Jeong YM, Kim B. Dual-source abdominopelvic computed tomography: Comparison of image quality and radiation dose of 80 kVp and 80/150 kVp with tin filter. PLoS One. 2020;15:e0231431. https://doi.org/10.1371/journal.pone.0231431.
Kanal KM, Butler PF, Sengupta D, Bhargavan-Chatfield M, Coombs LP, Morin RL. U.S. Diagnostic reference levels and achievable doses for 10 adult CT examinations. Radiology. 2017;284:120–33. https://doi.org/10.1148/radiol.2017161911.
Potter CA, Sodickson AD. Dual-energy CT in emergency neuroimaging: added value and novel applications. Radiographics. 2016;36:2186–98. https://doi.org/10.1148/rg.2016160069.
Gibney B, Redmond CE, Byrne D, Mathur S, Murray N. A review of the applications of dual-energy CT in acute neuroimaging. Can Assoc Radiol J. 2020;71:253–65. https://doi.org/10.1177/0846537120904347.
Funding
Open access funding provided by University of Zurich
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
R. Grkovski, L. Acu, U. Ahmadli, D. Nakhostin, P. Thurner, L. Wacht, Z. Kulcsár, H. Alkadhi and S. Winklhofer declare that they have no competing interests.
Ethical standards
This study was approved by the local ethics committee of the University Hospital Zurich (approval number 2018-01212). The study was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. All participants of the study gave written informed consent prior to examination. All participants gave approval for the data to be published.
Additional information
Risto Grkovski and Leyla Acu shared first authorship.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Grkovski, R., Acu, L., Ahmadli, U. et al. Dual-Energy Computed Tomography in Stroke Imaging. Clin Neuroradiol 33, 747–754 (2023). https://doi.org/10.1007/s00062-023-01270-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00062-023-01270-6