Abstract
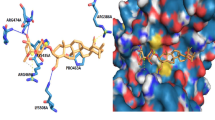
Breast and ovarian cancer are the most common cancers in women. Available cancer treatments, in general, have limited efficacy and frequent, undesirable side effects. Recently, scientists have focused on searching for new epigenetic modulators such as inhibitors of DNA methyltransferases and histone deacetylases (HDACs), with novel properties and selectivity. We report the synthesis of seven new analogs of Santacruzamate A. Molecular modeling showed that compounds 3–9 presented the best binding energies (kcal/mol) against HDAC4 compared to that of crystallographic ligand. The compounds were evaluated against MCF-7 and MDA-MB-231 (breast cancer), TOV-21G (ovarian adenocarcinoma), and WI-26VA4 (non-tumor lung fibroblasts) cells. Compound 5, the most potent and selective of the series, exhibited remarkably enhanced anticancer potency, with IC50 values for the tumor cells of 24.3–44.93 μM, compared with that of etoposide (12–18.57 μM) and doxorubicin (2.1–4.37 μM). Further investigation showed that compound 5 could promote DNA damage, increase the activity of caspases-3 and -9, and upregulate mRNA levels of p21, TP53, and BAK, suggesting apoptotic cell death of the tumor cells via the intrinsic pathway. This study demonstrated that synthetic analogs of santacruzamate A with zinc-linked groups are effective for improving both HDAC inhibition and antitumor activity.













Similar content being viewed by others
References
Adams JL, Smothers J, Srinivasan R, Hoos A (2015) Big opportunities for small molecules in immuno-oncology. Nat Rev Drug Discov 14:603–621. https://doi.org/10.1038/nrd4596
Ahn MY, Kang DO, Na YJ, Yoon S, Choi WS, Kang KW, Chung HY, Jung JH, Min DS, Kim HS (2012) Histone deacetylase inhibitor, apicidin, inhibits human ovarian cancer cell migration via class II histone deacetylase 4 silencing. Cancer Lett 325:189–199. https://doi.org/10.1016/j.canlet.2012.06.017
American Cancer Society (2018) Cancer facts & figures 2018. Am Cancer Soc. https://doi.org/10.3322/caac.21442
Berman HM, Kleywegt GJ, Nakamura H, Markley JL (2013) The future of the protein data bank. Biopolymers 99:218–222. https://doi.org/10.1002/bip.22132
Bolden JE, Shi W, Jankowski K, Kan CY, Cluse L, Martin BP, MacKenzie KL, Smyth GK, Johnstone RW (2013) HDAC inhibitors induce tumor-cell-selective pro-apoptotic transcriptional responses. Cell Death Dis 4:e-519. https://doi.org/10.1038/cddis.2013.9
Bonfils C, Kalita A, Dubay M, Siu LL, Carducci MA, Reid G, Martell RE, Besterman JM, Li Z (2008) Evaluation of the pharmacodynamic effects of MGCD0103 from preclinical models to human using a novel HDAC enzyme assay. Clin Cancer Res 14:3441–3449. https://doi.org/10.1158/1078-0432.CCR-07-4427
Carmichael J, Degraff WG, Gazdar AF, Minna JD, Mitchell JB (1987) Evaluation of a tetrazolium-based semiautomated colorimetric assay: assessment of chemosensitivity testing evaluation of a tetrazolium-based semiautomated colorimetrie assay: assessment. Am Assoc Cancer Res 47:936–942
Chen YJ, Wang WH, Wu WY, Hsu CC, Wei LR, Wang SF, Hsu YW, Liaw CC, Tsai WC (2017) Novel histone deacetylase inhibitor AR-42 exhibits antitumor activity in pancreatic cancer cells by affecting multiple biochemical pathways PLoS One 12:e0183368. https://doi.org/10.1371/journal.pone.0183368
Chen Y, Lopez-Sanchez M, Savoy DN, Billadeau DD, Dow GS, Kozikowski AP (2008) A series of potent and selective, triazolylphenyl-based histone deacetylases inhibitors with activity against pancreatic cancer cells and Plasmodium falciparum. J Med Chem 51:3437–3448. https://doi.org/10.1021/jm701606b
Custer LL, Sweder KS (2008) The role of genetic toxicology in drug discovery and optimization. Curr Drug Metab 9:978–985. https://doi.org/10.2174/138920008786485191
Damaskos C, Valsami S, Kontos M, Spartalis E, Kalampokas T, Kalampokas E, Athanasiou A, Moris D, Daskalopoulou A, Davakis S, Tsourouflis G, Kontzoglou K, Perrea D, Nikiteas N, Dimitroulis D (2017) Histone deacetylase inhibitors: an attractive therapeutic strategy against breast cancer. Anticancer Res 37:35–46. https://doi.org/10.21873/anticanres.11286
de Oliveira JT, da Silva Barbosa MC, de Camargos LF, da Silva IVG, de Pilla Varotti F, da Silva LM, Moreira LM, Lyon JP, da Silva Vieira dos Santos VJ, dos Santos FV (2017) Digoxin reduces the mutagenic effects of Mitomycin C in human and rodent cell lines. Cytotechnology 69:699–710. https://doi.org/10.1007/s10616-017-0078-3
Eastmond DA, Tucker JD (1989) Identification of aneuploidy‐inducing agents using cytokinesis‐blocked human lymphocytes and an antikinetochore antibody. Environ Mol Mutagen 13:34–43. https://doi.org/10.1002/em.2850130104
Fenech M (2000) The in vitro micronucleus technique. Mutat Res - Fundam Mol Mech Mutagen 455:81–95. https://doi.org/10.1016/S0027-5107(00)00065-8
Fenech M (2007) Cytokinesis block micronucleus cytome assay. Nat Protoc 2:1–35. https://doi.org/10.1038/nprot.2007.77
Formigli L, Papucci L, Tani A, Schiavone N, Tempestini A, Orlandini GE, Capaccioli S, Orlandini SZ (2000) Aponecrosis: morphological and biochemical exploration of a syncretic process of cell death sharing apoptosis and necrosis. J Cell Physiol 182:41–49. https://doi.org/10.1002/(SICI)1097-4652(200001)182:1<41::AID-JCP5>3.0.CO;2-7
Gandhi AK, Shi T, Li M, Jungnelius U, Romano A, Tabernero J, Siena S, Schafer PH, Chopra R (2013) Immunomodulatory effects in a phase II study of lenalidomide combined with cetuximab in refractory KRAS-mutant metastatic colorectal cancer patients. PLoS One 8: https://doi.org/10.1371/journal.pone.0080437
Gradishar WJ, Anderson BO, Balassanian R, Blair SL, Burstein HJ, Cyr A, Elias AD, Farrar WB, Forero A, Giordano SH, Goetz M, Goldstein LJ, Hudis CA, Isakoff SJ, Marcom PK, Mayer IA, McCormick B, Moran M, Patel SA, Pierce LJ, Reed EC, Salerno KE, Schwartzberg LS, Smith KL, Smith ML, Soliman H, Somlo G, Telli M, Ward JH, Shead DA, Kumar R (2015) NCCN guidelines insights breast cancer, version 1.2016. J Natl Compr Canc Netw 13:1475–85. https://doi.org/10.6004/JNCCN.2015.0176
Gromek SM, DeMayo JA, Maxwell AT, West AM, Pavlik CM, Zhao Z, Li J, Wiemer AJ, Zweifach A, Balunas MJ (2016) Synthesis and biological evaluation of Santacruzamate A analogues for anti-proliferative and immunomodulatory activity. Bioorg Med Chem 24:5183–5196. https://doi.org/10.1016/j.bmc.2016.08.040
Heberlé G, de Azevedo WF (2011) Bio-inspired algorithms applied to molecular docking simulations. Curr Med Chem 18:1339–1352. https://doi.org/10.2174/092986711795029573
Hsu YF, Sheu JR, Hsiao G, Lin CH, Chang TH, Chiu PT, Wang CY, Hsu MJ (2011) p53 in trichostatin A induced C6 glioma cell death. Biochim Biophys Acta - Gen Subj 1810:504–513. https://doi.org/10.1016/j.bbagen.2011.02.006
Hwang MH, Li XJ, Kim JE, Jeong SY, Lee SW, Lee J, Ahn BC (2015) Potential therapeutic effect of natural killer cells on doxorubicin-resistant breast cancer cells in vitro. PLoS One 10:e0136209. https://doi.org/10.1371/journal.pone.0136209
Kajstura M, Halicka HD, Pryjma J, Darzynkiewicz Z (2007) Discontinuous fragmentation of nuclear DNA during apoptosis revealed by discrete “Sub-G1” peaks on DNA content histograms. Cytom Part A 71:125–131. https://doi.org/10.1002/cyto.a.20357
Kalanxhi E, Risberg K, Barua IS, Dueland S, Waagene S, Andersen SN, Pettersen SJ, Lindvall JM, Redalen KR, Flatmark K, Ree AH (2017) Induction of apoptosis in intestinal toxicity to a histone deacetylase inhibitor in a phase I study with pelvic radiotherapy. Cancer Res Treat 49:374–386. https://doi.org/10.4143/crt.2016.080
Kolitz JE, George SL, Dodge RK, Hurd DD, Powell BL, Allen SL, VelezGardcia E, Moore JO, Shea TC, Hoke E, Caligiuri MA, Vardiman JW, Bloomfield CD, Larson RA (2004) Dose escalation studies of cytarabine, daunorubicin, and etoposide with and without multidrug resistance modulation with PSC-833 in untreated adults with acute myeloid leukemia younger than 60 years: final induction results of cancer and leukemia group B. J Clin Oncol 22:4290–4301. https://doi.org/10.1200/JCO.2004.11.106
Koopman BG, Reutelingsperger CPM, Kuijten GAM, Keehnen RMJ, Pals ST, van Oers MHJ (1994) Annexin V for flow cytometric detection of phosphatidylserine expression on B cells undergoing. Apoptosis 3:11–12
Kulshrestha A, Katara GK, Ibrahim S, Pamarthy S, Jaiswal MK, Sachs AG, Beaman KD (2015) Vacuolar ATPase “a2” isoform exhibits distinct cell surface accumulation and modulates matrix metalloproteinase activity in ovarian cancer. Oncotarget 6:3797–810. https://doi.org/10.18632/oncotarget.2902
Liu Z, Ding K, Li L, Liu H, Wang Y, Liu C, Fu R (2016) A novel histone deacetylase inhibitor chidamide induces G0/G1 arrest and apoptosis in myelodysplastic syndromes. Biomed Pharmacother 83:1032–1037. https://doi.org/10.1016/j.biopha.2016.08.023
Manal M, Chandrasekar MJN, Gomathi Priya J, Nanjan MJ (2016) Inhibitors of histone deacetylase as antitumor agents: a critical review. Bioorg Chem 67:18–42. https://doi.org/10.1016/j.bioorg.2016.05.005
Marx-blümel L, Marx C, Kühne M, Sonnemann J (2017) HDAC/HAT function assessment and inhibitor development. 1510: https://doi.org/10.1007/978-1-4939-6527-4
Musa MA, Latinwo LM, Joseph MY, Badisa VL (2017) Identification of 7,8-diacetoxy-3-arylcoumarin derivative as a selective cytotoxic and apoptosis-inducing agent in a human prostate cancer cell line. Anticancer Res 37:6005–6014. https://doi.org/10.21873/anticanres.12047
Nunes RR, dos Santos Costa M, dos Reis Santos B, da Fonseca AL, Ferreira LS, Chagas RCR, da Silva AM, de Pilla Varotti F, Taranto AG (2016) Successful application of virtual screening and molecular dynamics simulations against antimalarial molecular targets. Mem Inst Oswaldo Cruz 111:721–730. https://doi.org/10.1590/0074-02760160207
Ohkoshi SI, Namai A, Imoto K, Yoshikiyo M, Tarora W, Nakagawa K, Komine M, Miyamoto Y, Nasu T, Oka S, Tokoro H (2015) Nanometer-size hard magnetic ferrite exhibiting high optical-transparency and nonlinear optical-magnetoelectric effect. Sci Rep 5:1–9. https://doi.org/10.1038/srep14414
Olaharski AJ, Ji Z, Woo JY, Lim S, Hubbard AE, Zhang L, Smith MT (2006) The histone deacetylase inhibitor trichostatin a has genotoxic effects in human lymphoblasts in vitro. Toxicol Sci 93:341–347. https://doi.org/10.1093/toxsci/kfl068
Park KS, Frost B, Tuck M, Ho LL, Kim S, Paik WK (1987) Enzymatic methylation of in vitro synthesized apocytochrome c enhances its transport into mitochondria. J Biol Chem 262:14702–14708
Parsa Y, Mirmalek SA, Kani FE, Aidun A, Salimi-tabatabaee SA, Yadollah-damavandi S, Jangholi E, Parsa T, Shahverdi E, Researchers Y, Club E, Medical T, Branch S, Medical T, Branch S, Medical T, Branch S (2016) Electronic physician. 2416–2424. https://doi.org/10.14661/1412
Pavlik CM, Wong CY, Ononye S, Lopez DD, Engene N, McPhail KL, Gerwick WH, Balunas MJ (2013) Santacruzamate A, a potent and selective histone deacetylase inhibitor from the panamanian marine cyanobacterium cf. symploca sp. J Nat Prod 76:2026–2033. https://doi.org/10.1021/np400198r
Petros AM, Olejniczak ET, Fesik SW (2004) Structural biology of the Bcl-2 family of proteins. Biochim Biophys Acta - Mol Cell Res 1644:83–94. https://doi.org/10.1016/j.bbamcr.2003.08.012
Randino R, Gazzerro P, Mazitschek R, Rodriquez M (2017) Synthesis and biological evaluation of santacruzamate-A based analogues. Bioorganic. Med Chem 25:6486–6491. https://doi.org/10.1016/j.bmc.2017.10.026
Rane CK, Minden A (2014) P21 activated kinases: structure, regulation, and functions. Small GTPases. 1–13, pii: e28003. https://doi.org/10.4161/sgtp.28003
Rephaeli A, Waks-Yona S, Nudelman A, Tarasenko I, Tarasenko N, Phillips DR, Cutts SM, Kessler-Icekson G (2007) Anticancer prodrugs of butyric acid and formaldehyde protect against doxorubicin-induced cardiotoxicity. Br J Cancer 96:1667–1674. https://doi.org/10.1038/sj.bjc.6603781
Rexer BN, Arteaga CL (2012) Intrinsic and acquired resistance to HER2-targeted therapies in HER2 gene-amplified breast cancer: mechanisms and clinical implications. Crit Rev Oncog 17:1–16. https://doi.org/10.1615/CritRevOncog.v17.i1.20
Rose NR, Ng SS, Mecinović J, Liénard BMR, Bello SH, Sun Z, McDonough MA, Oppermann U, Schofield CJ (2008) Inhibitor scaffolds for 2-oxoglutarate-dependent histone lysine demethylases. J Med Chem 51:7053–7056. https://doi.org/10.1021/jm800936s
Roy S, Packman K, Jeffrey R, Tenniswood M (2005) Histone deacetylase inhibitors differentially stabilize acetylated p53 and induce cell cycle arrest or apoptosis in prostate cancer cells. Cell Death Differ 12:482–491. https://doi.org/10.1038/sj.cdd.4401581
Stewart JJP (2016) MOPAC2016TM. http://openmopac.net/MOPAC2016.html
Swift LH, Golsteyn RM (2014) Genotoxic anti-cancer agents and their relationship to DNA damage, mitosis, and checkpoint adaptation in proliferating cancer cells. Int J Mol Sci 15:3403–3431. https://doi.org/10.3390/ijms15033403
Terranova-Barberio M, Thomas S, Ali N, Pawlowska N, Park J, Krings G, Rosenblum MD, Budillon A, Munster PN (2017) HDAC inhibition potentiates immunotherapy in triple negative breast cancer. Oncotarget 8:114156–114172. https://doi.org/10.18632/oncotarget.23169
Trott O, Olson A (2010) NIH public access. J Comput Chem 31:455–461. https://doi.org/10.1002/jcc.21334.AutoDock
Tryfonidis K, Zardavas D, Katzenellenbogen BS, Piccart M (2016) Endocrine treatment in breast cancer: cure, resistance and beyond. Cancer Treat Rev 50:68–81. https://doi.org/10.1016/j.ctrv.2016.08.008
Utani KI, Kohno Y, Okamoto A, Shimizu N (2010) Emergence of micronuclei and their effects on the fate of cells under replication stress. PLoS One 5: https://doi.org/10.1371/journal.pone.0010089
Venkatesh S, Workman JL (2015) Histone exchange, chromatin structure and the regulation of transcription. Nat Rev Mol Cell Biol 16:178–189. https://doi.org/10.1038/nrm3941
Volkmann N, Marassi FM, Newmeyer DD, Hanein D (2014) The rheostat in the membrane: BCL-2 family proteins and apoptosis. Cell Death Differ 21:206–215. https://doi.org/10.1038/cdd.2013.153
Wang Y, Xu Y, Chen J, Ouyang T, Li J, Wang T, Fan Z, Fan T, Lin B, Xie Y (2016) TP53 mutations are associated with higher rates of pathologic complete response to anthracycline/cyclophosphamide-based neoadjuvant chemotherapy in operable primary breast cancer. Int J Cancer 138:489–496. https://doi.org/10.1002/ijc.29715
World Health Oganization (WHO) (2015) IARC handbooks on breast cancer screening (2015). http://www.who.int/cancer/prevention/diagnosis-screening/breast-cancer/en/
Yano M, Yasuda M, Sakaki M, Nagata K, Fujino T, Arai E, Hasebe T, Miyazawa M, Miyazawa M, Ogane N, Hasegawa K, Narahara H (2018) Association of histone deacetylase expression with histology and prognosis of ovarian cancer. Oncol Lett 15:3524–3531. https://doi.org/10.3892/ol.2018.7726
Zghair AN, Sinha DK, Kassim A, Alfaham M, k Sharma A (2016) Differential gene expression of BRCA1,ERBB2 and TP53 biomarkers between human breast tissue and peripheral blood samples of breast cancer patients. Anticancer Agents Med Chem 7:5519–525
Zhang J, Zhong Q (2014) Histone deacetylase inhibitors and cell death. Cell Mol Life Sci 71:3885–3901. https://doi.org/10.1007/s00018-014-1656-6
Zhang P, Chen J, Liang Y (2010) DNA binding, cytotoxicity, and apoptotic-inducing activity of ruthenium(II) polypyridyl complex. Acta Biochim Biophys Sin 42:440–449. https://doi.org/10.1093/abbs/gmq040.Advance
Zhou Q, Eldakhakhny S, Conforti F, Crosbie EJ, Melino G, Sayan BS (2018) Pir2 / Rnf144b is a potential endometrial cancer biomarker that promotes cell proliferation. Cell Death Dis 1–10. https://doi.org/10.1038/s41419-018-0521-1
Acknowledgements
The authors would like to acknowledge the financial support received from the following institutions: Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG) FAPEMIG and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
Author information
Authors and Affiliations
Contributions
SNA, FCGE, DRM, TRF, JTO, RIMAR, HBS, RGT and FVS performed the biological experiments. DS, GHRV, RPF and JLH performed the synthesis and structural elucidation. RRN and AGT performed the in silico approach. APS, FFH and FPV coordinated the research. All authors wrote the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Andrade, S.N., Evangelista, F.C.G., Seckler, D. et al. Synthesis, cytotoxic activity, and mode of action of new Santacruzamate A analogs. Med Chem Res 27, 2397–2413 (2018). https://doi.org/10.1007/s00044-018-2244-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-018-2244-3