Abstract
In the present paper, sedimentological, water physicochemical data and biological benthic assemblages are compiled to provide an integrated environmental assessment of a protected shallow Mediterranean lagoon with a long history of management interventions (Klisova Lagoon, Western Greece). Satellite remote sensing techniques were used for mapping the seagrass Cymodocea nodosa (Ucria) Asch, which presents an extensive expansion in the study area. The results showed that Klisova is a hypersaline, well-oxygenated lagoon presenting changes in temperature, salinity, pH and dissolved oxygen over the course of 1 year, but largely lacks any spatial differentiation other than an east–west gradient in salinity in spring and summer. Sediment was classified as silty sand to silt, rich in total organic carbon (TOC), total nitrogen (TN) and total phosphorus (TP). The benthic community was characterised by a high species richness and the presence of marine species, without a clear spatial pattern. The multivariate-AZTI’s marine biotic index (M-AMBI) showed that the ecological quality of the macrobenthos was good in most stations. C. nodosa covered over 70% of the lagoon area and formed meadows even in the eastern part of the lagoon where salinity during spring and summer was close to the reported upper tolerance limit of the species (> 50). The present multidisciplinary approach provides valuable baseline information on various components of the ecosystem which can be used as a basis for management bodies and future conservation plans for the lagoon.
Similar content being viewed by others
Introduction
The transitional zones between freshwater and marine environments, such as lagoons, coastal marshes, estuaries and deltas, constitute sensitive ecosystems with unique biological, geological and chemical properties. Such ecosystems, which are included among the most productive ecosystems in the world, provide a variety of direct and indirect ecosystem goods and services, such as nutrient processing, aquatic food production, recreational activities and aesthetics (Basset et al. 2013).
Mediterranean coastal lagoons are generally shallow, confined bodies of brackish or hypersaline water that develop with their central axis parallel to the coasts and are separated from the open sea by characteristic sandy or gravelly barriers (Kjerfve 1994; Bird 2011). These coastal barriers comprise a distinct feature of lagoonal systems from other similar coastal systems, such as estuaries (Nichols and Allen 1981), and limit the connection of these systems with the sea, which takes place via one or more shallow inlet (Phleger 1981). The degree of this confinement, along with the other geographical and topographical features of each lagoon, leads to the development of strong spatial and seasonal gradients of the abiotic properties (e.g. temperature, pH, dissolved oxygen, conductivity, etc.) which in turn affect the productivity, diversity and distribution patterns of the fauna and flora (Nicolaidou et al. 2005).
During the last two decades, a lot of scientific effort has been invested in the typology of these highly heterogeneous and variable ecosystems towards the grouping of transitional waters (TWs) and the definition of ecological units that are more easily managed (Nicolaidou et al. 2005; Barbone et al. 2012; Basset et al. 2013). Although common key elements that drive the functionality of TWs have been identified (i.e. salinity, confinement, tidal regime) (Basset et al. 2006), knowledge of the specific hydrogeological and biological features of each lagoon is imperative for the conservation and recovery of good ecological status under the Water Framework Directive (WFD), as well as for managerial purposes. The monitoring of lagoon systems is also vital as most lagoons face threats from anthropogenic impacts, including climate change, eutrophication and changes in the hydrological regime (Basset et al. 2013). Thus, adequate knowledge of their abiotic and biotic characteristics is crucial for decision making and preservation of their important roles for ecology, society and economy.
As part of the monitoring of TWs under the WFD, macrobenthic communities are one of the biotic quality elements used to assess ecological status. Macrobenthic organisms are often considered highly adequate descriptors of the ecosystem condition owing to their mostly sedentary lifestyle and their reaction to changes in the environment. They have also been extensively studied as indicators for water quality assessment for many decades (e.g. Simboura and Reizopoulou 2008). A variety of biotic indices have been proposed towards this end, such as AZTI’s marine biotic index (AMBI, Borja et al. 2000), multivariate-AMBI (M-AMBI) (Muxika et al. 2007), BENTIX (Simboura & Zenetos 2002), benthic opportunistic annelida amphipod index (BO2A; Dauvin & Ruellet 2007), index of size distribution (ISD; biomass size structure index, Reizopoulou & Nicolaidou 2007) or index of size spectra sensitivity index (ISS; Basset et al. 2012) (see Cabana et al. 2013 for a detailed overview). Many of these indices have been evaluated in different regions and countries and have been found to locally differ in their effectiveness in detecting anthropogenic disturbance (e.g. Reizopoulou et al. 2018, Magni et al 2023; Barbone et al. 2012). In most Mediterranean EU states, including Greece, the M-AMBI index has been adopted for the purpose of monitoring transitional waters (Reizopoulou et al. 2018). It is a multi-metric index based on the original AMBI index, but including species richness and Shannon diversity. One of its major strengths is that it allows for the definition of different reference conditions for different habitats. This makes the M-AMBI especially suitable for lagoons which usually present very idiosyncratic conditions as a result of their geomorphological, sedimentary, meteorological and physicochemical characteristics (Kjerfve 1986).
Seagrass species in lagoons constitute a key component regarding ecosystem stability, resilience and biodiversity (Pergent et al. 2002; Signorini et al. 2008). In parallel, seagrass species are especially vulnerable to various stress factors (natural or anthropogenic) which cause regression to their expansion (Boudouresque et al. 2009) and, as such, the mapping of their distribution is highly important.
The present study aims to describe and characterise several environmental and ecological components of Klisova Lagoon, which belongs to one of the most important Greek wetlands, the Messolonghi–Aitoliko–Klisova lagoons complex. We combine data concerning the physicochemical regime of the water with multi-proxy techniques of sedimentology, macrozoobenthic analyses and remote sensing for mapping the C. nodosa meadows in the lagoon to provide baseline information and demonstrate an integrated approach useful for management bodies and future conservation plans for the lagoon.
Study area
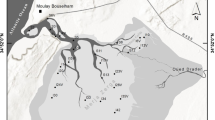
The study was conducted in Klisova Lagoon, located in western Greece and belonging to the extensive wetland of the Messolonghi–Aitoliko–Klisova lagoonal complex (Fig. 1). It is protected by the Ramsar Convention and listed in the Natura 2000 network (code GR2310001). The lagoon of west Klisova is economically important as it is traditionally exploited for fishing, mainly by means of barrier traps, having an average annual production of 22 mt which mostly comprises the species Liza aurata, Liza saliens, Mugil cephalus, Sparus aurata, Anguilla anguilla and Dicentrarchus labrax (Katselis et al. 2003). The lagoon is also ecologically important as it is a nursing area for the Dalmatian pelican Pelecanus crispus among other bird species.
The Messolonghi–Aitoliko–Klisova lagoonal complex (left) and the study area (right). Black and blue circles: sampling stations for physicochemical characteristics and sediment samples. Blue circles: sampling stations for macrofaunal samples and Cymodocea shoot collection. Arrows indicate the water exchange channels
Klisova Lagoon is a typical shallow Mediterranean brackish lagoon with 1 m mean depth (maximum 1.5 m). It has formed and evolved during the last 4000 years before the present (BP) in response to Evinos River discharge and delta progradation, combined with climatic conditions and anthropogenic activity (Emmanouilidis et al. 2022). The lagoon was separated by the main Messolonghi–Aitoliko lagoon complex in 1885 by the construction of a road in its western part. In the late 1960s, the lagoon was separated artificially into West Klisova of approximately 1900 ha and East Klisova of 600 ha (Fig. 1). Water exchange with the sea in West Klisova takes place directly through two major channels (30 m width, 2 m depth) at its southern part and indirectly through three smaller channels (10 m width, 2 m depth) along its western side (Fig. 1). In the 1970s, to facilitate the operation of the two adjacent salt work companies, the interior of the lagoon was divided into smaller parts through the construction of soil barriers that restricted water circulation and consequently led to increased salinity. Along the barriers openings with varying widths ranging from 8 to 25 m existed, which facilitated water exchange between the artificial ‘compartments’ in the lagoon. In parallel, all freshwater inputs in the lagoon were cut off (i.e. streams and artificial canals) and significant amounts of the hypersaline water from the lagoon is being pumped towards the saltworks and replenished by seawater during the warm period of the year. Thus, salinity in the study area has been reported to range between 30 psu (spring) and 55 psu (late summer) during the year in its western part, whereas in the more isolated northern and eastern parts, salinity often exceeds 70 (Hotos and Avramidou 1997). In the early 2000s, some of the soil barriers were removed to improve water circulation and, since then, three separate parts can be defined in the lagoon (i.e. northern, central and southern) by artificial or natural barriers which restrict water circulation to varying extents. The salinity regime of the lagoon seems to be controlled by two main processes: on the one hand, the existence of the internal barriers restricts water circulation in the lagoon which results in increased salinity. On the other hand, the pumping stations of the saltworks draw significant amounts of high-saline waters from Klisova (approximately 8 million m−3 during the warm season; Seretaki 2012 – Technical report, in Greek) which is replenished by the lower salinity water of Patraikos Gulf (38–39, Ramfos et al. 2005). Historically, Klisova is referred to as a hypersaline lagoon with values ranging around 40 in the western part and close to 90 in the more isolated parts of the northern and eastern parts during summer months (Chatzikakidis 1953; Hotos and Avramidou 1997). Although there are fragmentary observations during the last decades, it is possible that the partial removal of the barriers in 2005 improved water circulation in the lagoon and prevented the development of the extreme salinity values that have been observed in the past.
Although some parts of the Messolonghi–Aitoliko–Klisova lagoon complex suffered several times in the past from hypoxic and anoxic events, especially in Aitoliko and East Klisova lagoons (Avramidis et al. 2010, 2015, 2017; Gianni and Zacharias 2012), there is no record of such events for West Klisova. During the last 25 years, studies have described several environmental aspects of Klisova lagoon, covering heavy metal concentrations in sediments (Papatheodorou et al. 2002; Marazioti et al. 2010; Karageorgis et al. 2012), water physicochemical parameters, macrophytes and seagrasses (Hotos and Avramidou 1997; Christia and Papastergiadou 2007; Christia et al. 2018; Orfanidis et al. 2020).
Materials and methods
Sampling and field measurements
The physicochemical characteristics of the water column in West Klisova were monitored seasonally during one year (July and November 2016, January and March 2017). The sampling grid comprised 20 stations (1–20) and one sample was collected in each station per season (Fig. 1). Sediment samples were collected from the same stations in summer 2016. Positioning was achieved with a hand-held GPS (accuracy 2 m). Seasonal physicochemical measurements such as pH, salinity, temperature (T, ℃) and dissolved oxygen (DO, mg/L) were measured in situ with a portable HACH HQ40D (Loveland, Colorado, USΑ) instrument. In each monitoring station, surficial sediment samples were collected using a Van Veen grab with a sampling surface of 0.025 m. A subsample from the top 5 cm of sediment was collected for sediment analysis.
During the sampling of March 2017, macrofaunal samples were collected at seven of the stations (i.e. 1, 2, 4, 6, 8, 9 and 15) covering, as much as possible, the different parts of the lagoon (Fig. 1). In each station, three separate replicates were taken with a Van Veen grab (0.025 m2 sampling surface). The collected material was sieved through a 0.5 mm mesh and the remaining material was fixed in 4% formaldehyde buffered with seawater. Both the time of sampling (spring) as well as the sampling methodology follow the protocol of the national benthic macroinvertebrate monitoring of transitional waters for the Water Framework Directive, thus the results of this study are comparable with those data.
Finally, during summer 2016 when C. nodosa presents its highest shoot density (Zavodnik et al. 1998), the presence/absence of C. nodosa meadow was visually confirmed in all sampling stations, as well as along the routes between sampling stations. Presence/absence of C. nodosa was also noted during sampling for macrobenthos. Additionally, to obtain an indication of the shoot density of the meadows, shoots were collected in stations 1, 2, 4, 6, 8, 9 and 15 by grab sampling. In the cases of vegetated sediment, the grab was gently pushed by hand into the sediment to penetrate through the rhizomes and the collected shoots were rinsed and counted in the laboratory.
Sedimentology
Sediment samples were analysed for granulometric and chemical analysis. Material coarser than 4 Φ (> 2 mm) was dried and sieved, fine-grained material (< 2 mm) was analysed using a Malvern Mastersizer 2000, and grain size distribution was calculated. Colours were identified using a Minolta CM-2002 hand-held spectrophotometer on the basis of the Munsell colour chart. The sediment’s total organic carbon (TOC) content was determined by the titration method (Gaudette et al. 1974; Avramidis and Bekiari 2021), and total nitrogen (TN) with a CHNS-O EA 1108 Elemental Analyzer by Carlo Erba. Total phosphorus (TP) was determined on the basis of the APHA 2005–4500-P method with a spectrophotometer DR 3800 HACH-LANGE. Carbonates (CaCO3) were measured on the basis of Müller and Gastner (1971) and Jones and Kaiteris (1983) using a FOG II/Digital calcimeter, version 2/2014 (BD INVENTIONS).
Macrobenthic assemblages
Macrobenthic samples were washed to remove excess formaldehyde, and organisms were extracted from the sediment residues under a stereomicroscope and transferred to 70% ethanol. All animals were identified to species level and their abundance was counted; in cases where this was not possible owing to poor condition of the organism or very small size, taxa were assigned to the next highest taxonomic level. The most recent taxonomic literature was used for identification, and all taxonomic names were cross-checked against the World Register for Marine Species (WoRMS Editorial Board 2022).
For the analyses of macrofaunal data, replicates were pooled and their abundances averaged per station. Abundance data were standardised to densities (individuals m−2). Biodiversity indices [number of individuals, number of taxa, Shannon–Wiener index Η’ (Shannon 1948), Pielou’s index, but not the M-AMBI index] were calculated on the pooled and averaged data. To estimate the ecological quality of the macrobenthos, the M-AMBI index was calculated (Muxika 2007). The M-AMBI index is a multivariate index which incorporates species richness, the Shannon diversity index and the AMBI index (Borja et al. 2000). The AMBI index is based on an approach where taxa are assigned to one of five ecological groups (EG), on the basis of their sensitivity to disturbance. The proportions of each group in the sample are calculated and each proportion is multiplied with a modifier. This results in a number between 0 (only sensitive species present, pristine conditions) and 6 (only highly tolerant species present, highly polluted conditions) and resulting in 7 when the sediment is azoic. The M-AMBI index then uses factor analyses which incorporates the three metrics, but also requires the user to specify reference values for the ‘high’ (i.e. unpolluted) and ‘bad’ (i.e. highly polluted) conditions. Since Klisova Lagoon is considered a euhaline restricted lagoon in accordance with national River Basin Management Plans, reference values for the calculation of the M-AMBI index and its boundaries follow those in Reizopoulou et al. (2018) and were set as follows: reference for ‘high’ status: AMBI = 0.05; H’ = 4; S = 50; boundaries between classes: high: > 0.83; good: > 0.62–0.83; moderate: > 0.41–0.62; poor: 0.20–0.41; bad: < 0.20).
To assess the relatedness of the sampling stations on the basis of the identity and abundance of macrofaunal organisms, data were square root-transformed to reduce the effect of very high abundances on the analyses, similarities between stations were calculated using the Bray–Curtis index (Bray and Curtis 1957) and a hierarchical dendrogram was plotted using the complete linkage method.
The macrofaunal multivariate dataset was correlated with the standardised physicochemical water parameters of both the winter and the spring sampling and with the sediment characteristics (separate analyses), to determine those abiotic parameters showing the highest correlation with the macrofaunal community data. A comparison was made both with the winter and the spring sampling to assess the correlation of the macrofaunal pattern with the environmental conditions at the time of sampling, but also determine potential effects that preceding conditions (i.e. in winter) might have had on the current condition of the macrofauna. The correlation was performed through the BIOENV analysis in the R package vegan 2.5–7 (Oksanen et al. 2020). All statistical analyses were performed in R version 4.1.2 with the exception of the M-AMBI index, for which the AMBI software (https://ambi.azti.es/) was used.
Cymodocea nodosa mapping
A Worldview-2 bundle image of 23 August 2015 (one panchromatic and eight multi-spectral bands) was acquired and processed. The panchromatic band has a spatial resolution of 0.46 m, while the multi-spectral bands have a spatial resolution of 1.84 m. The Worldview-2 image comprises four typical visible and near-infrared bands similar to previous multi-spectral satellites, such as Ikonos Quickbird, Geoeye Landsat-7 and four novel bands. These new spectral channels permit the user to distinguish very small existing spectral differences into the image, which might be difficult to observe in traditional multi-spectral imagery. The processing of the Worldview data was performed in ERDAS Imagine software in three steps: (1) fusion of panchromatic and multi-spectral data, (2) image orthorectification and (3) principal component analysis.
The panchromatic and the eight multi-spectral bands were fused to ameliorate the spatial resolution of the multi-spectral while at the same time keeping the spectral information invariable. According to the results of previous studies (Nikolakopoulos 2008; Nikolakopoulos and Oikonomidis 2015), the Pansharp algorithm was selected for the fusion of the panchromatic and multi-spectral data. The final produced image of the study area has eight bands with a spatial resolution of 0.46 m.
After the fusion, the Worldview-2 imagery was orthorectified in the Imagine Photogrammetry Suite of ERDAS Imagine Software using the relevant sensor (Worldview-2) model. The orthorectification procedure used ten ground control points and a very accurate digital surface model with 5 m spatial resolution. A double-frequency global navigation satellite system (GNSS) receiver was used to collect the ground control points (GCPs) used in the next stages for data processing and accuracy assessment. During the GCP measurements, the horizontal root mean square error (RMSE) was between 0.8 and 1.2 cm, whereas the vertical RMSE was between 1.5 and 2.1 cm. Therefore, the final RMSE of the orthorectification was lower than half of the image pixel size. Afterwards, a principal component analysis (PCA) was performed to extract the maximum information from the orthorectified Worldview-2 image. PCA is a mathematical transformation technique of the original image to a new set of uncorrelated components derived in decreasing order of importance by applying a Gaussian distribution (Kyriou and Nikolakopoulos 2020). The new image bands are more easily interpretable than the original ones.
Results
Physicochemical characteristics of the water column
Mean water temperature of all monitoring stations (1–20) in the four different sampling periods fluctuated between 15.1 and 29.3 °C (Table 1) with a yearly average temperature of 21 °C, whereas the minimum and maximum recorded temperature was 14.4 and 32.8 ℃, respectively. Salinity presented highest values in summer and lowest in winter but was overall high in all sampling stations: average salinity ranged from 36.9 to 52.9 psu (Table 1) with a yearly mean of 44.4 psu. The spatial distribution of salinity was more or less homogeneous during autumn and winter but presented a clear increasing trend along the west–east axis during spring and summer (Fig. 2). During summer, salinity in the western part of the lagoon ranged between 43.0 and 48.9 psu and increased towards the eastern part where it ranged from 58.8 to 67.2 psu. The lagoon was well oxygenated during all samplings. Mean dissolved oxygen (DO) values ranged between 6.2 mg L−1 (summer) and 8.76 mg L−1 (autumn) (Table 1). DO was above 5.0 mg L−1 during all sampling periods, with the exception of sites 11, 18 and 19 during summer where DO was 4.8, 4.9 and 3.5 mg L−1, respectively. Mean pH values presented lowest values in autumn and highest in spring (Table 1). Average pH during the sampling period ranged between 8.22 and 8.99.
Sediments grain size and nutrients
The lithological sediment types were estimated on the basis of Folk’s (1974) classification. The main lithological type of the lagoon sediments was very dark grey to very dark greyish-green, silt to sandy silt. The sand fraction ranged between 0.8% and 55%, the silt fraction ranged between 39% and 90%, while the clay fraction was < 10% in all samples. Total organic carbon (TOC) concentrations in the sediment ranged from 2.01% to 5.3% with a mean value of 3.71%, while total nitrogen (TN) concentration varied from 0.19% to 0.74% with a mean value of 0.36%. Total phosphorus (TP) concentration ranged from 103 to 373 ppm, with a mean value of 232 ppm. Carbonates fluctuated between 8.1% and 45% with a mean value of 24.8%. All chemical characteristics of the sediment presented a rather uniform spatial distribution in the lagoon without any obvious pattern (Table 2).
Macrobenthic assemblages
Overall, 71 taxa and 1197 individuals were found in the seven sampling stations during March 2017. The highest number of taxa was found in station 1 (42 species) and the lowest in station 2 (7 species; Table 3). The average number of individuals per m−2 varied from 213 in station 2 to 3973 in Station 8. Pielou’s evenness index ranged from 0.68 in station 9 to 0.93 in station 2. The Shannon–Wiener diversity index was highest at station 1 (4.64) and lowest at station 2 (2.60).
Polychaetes were the dominant taxon at all stations except for stations 8 and 9 where crustaceans were the most abundant taxon (Fig. 3). However, no single taxon dominated at any of the stations (the highest relative abundance was Microdeutopus sp. with 41.0% at station 9); this was also reflected in the relatively high evenness values of Pielou’s index.
Overall, the fauna was characterised by euryhaline and marine taxa, whereas only few individuals of typical brackish water taxa, such as Gammarus insensibilis, Idotea balthica, Naineris laevigata, Nephtys hombergii or oligochaetes and chironomid larvae were encountered. The finding of the fabriciid polychaete Novafabricia posidoniae Licciano and Giangrande 2006 is the second published record of this taxon in Greece (Faulwetter et al. 2017) and the first record for the Ionian Sea. The ecological status for benthic macroinvertebrates (expressed through the M-AMBI index) was good at all stations, except for station 1 which was classified as ‘High’ and station 2 which was classified as ‘Moderate’ (Table 3 and Fig. 4).
The hierarchical dendrogram of stations (Fig. 5) based on macrofaunal communities showed that station 2 was strongly different (> 80% dissimilarity) from other stations based on the community characteristics. All stations showed a relatively high dissimilarity (over 50%) to each other and only stations 8 and 9 presented a slightly higher similarity. No evident spatial clustering could be discerned in the dendrogram, apart from stations 8 and 9 which are spatially close to each other. The BIOENV analysis did not detect any notable correlations (ρ < 0.4 in all cases) between the macrofaunal community distribution and any abiotic parameters measured in the lagoon (Table 4).
Cymodocea nodosa mapping
The principal component analysis produced uncorrelated spectral bands which facilitated the detection of the hidden spectral information in the original unprocessed imagery. This revealed two areas with a characteristically different colour than the rest of the area which were characterised by the absence of C. nodosa (Fig. 6 pink colour) in contrast to the vegetated areas (Fig. 6 green colour). These two areas of bare sediments covered almost 7% of the total sediment surface of the lagoon. Visual and grab ground truthing confirmed the extensive distribution of C. nodosa in the lagoon as well as its absence in the two bare areas. Meadow density was not uniform and shoot density ranged among stations from 400 to 1600 shoots per m−2 with an average of 833 ± 536 shoots per m−2.
Discussion
Klisova has been categorised as an euhaline lagoon (Hotos and Avramidou 1997; Christia et al 2018) but according to this study, Klisova presents seasonally hypersaline conditions as salinity is higher than 40 during most of the year.
Despite the salinity gradient in the lagoon which was observed mainly in summer and to some extent in spring, all other physicochemical parameters in water and sediment presented a rather homogeneous distribution in the lagoon. Mediterranean lagoons typically show a strong environmental gradient from the sea towards the inner parts of the lagoon (Reizopoulou et al 1998; Reizopoulou and Nicolaidou 2004) which in turn shapes the biotic communities (i.e. the confinement theory established by Guelorget and Perthuisot 1983). Typically, communities closer to the opening of the lagoon to the sea are characterised by marine and euryhaline species, whereas species in the inner parts of the lagoons are composed of brackish and even freshwater species (Nicolaidou and Reizopoulou 2005). In this study, marine and euryhaline species dominated at all stations. This is likely owing to the fact that Klisova Lagoon does not receive freshwater input apart from rainfall and groundwater, and thus retains relatively high salinities in all sampling stations. In addition, the pumping mechanism of the saltworks creates an artificial hydrodynamic regime which increases water renewal rates from the sea, and thus affects the composition of macrobenthic communities.
The lack of a spatial structuring of the macrobenthic communities was also apparent from the clustering based on the Bray–Curtis coefficient. No spatial clustering was observed in the dendrogram (with the exception of the two neighbouring stations 8 and 9 which cluster together), and communities showed a high degree of dissimilarity. No correlation of macrobenthic communities with environmental parameters could be detected (see BIO-ENV analysis, Table 4), thus community composition seems to not be influenced by any of the measured water or sediment characteristics but possibly by highly localised conditions, such as different levels of habitat complexity formed by different types of vegetation, which have been shown to influence taxonomic and functional diversity (Verdonschot et al. 2012; St. Pierre and Kovalenko 2014; Cabana et al. 2017).
Especially the presence or absence of seagrass could play a role in shaping the benthic communities. Complex biogenic habitats, such as seagrass meadows, often host a higher biodiversity than uniform sediments (e.g. Barnes et al. 2022, Connolly 1997, Edgar 1994); however, it has also been shown that species richness and abundance in disturbed or fragmented biogenic habitats can be even higher than in undisturbed ones (e.g. Urra et al. 2013; Fahrig 2003; Boström et al. 2010). This can be explained by edge effects: the fact that communities at the boundaries between habitats are influenced by the different species pools of the individual habitats, leading to a higher biodiversity (e.g. Bologna et al. 2002, Tanner 2005).
In Klisova, the sampled stations hosted different macrofaunal communities with different characteristics. Station 2 was located on bare sediment and showed the lowest values of the Shannon–Wiener index, as well as the most dissimilar community composition compared with the other stations. Station 1, located at the edge of the meadow towards the bare area showed the highest macrofaunal diversity, which could be owing to an edge effect, as seen in other studies (Tanner 2005, Moore and Hovel 2010). Similarly, stations 8 and 9 show similar macrobenthic communities and are located in a similar habitat (sparse meadow) but are also located spatially close to each other. Again, these effects cannot be disentangled easily. Overall, as no strong correlations with environmental parameters nor any obvious spatial grouping could be detected, the characteristics and/or complexity of the Cymodocea meadow (both in terms of density and patchiness/proximity to edges) may be a factor structuring these communities. In addition, it has been found that lagoons dominated by seagrasses may not show any confinement of the macrobenthic communities, as these systems can influence water renewal times and local organic enrichment (Barnes 2023).
The macrozoobenthic community sampled in spring in Western Klisova showed a high species richness overall, except at station 1. The near-undisturbed reference conditions for a restricted euryhaline lagoon in Greece have been estimated to have a species richness of 50 and a Shannon index of 4 (Reizopoulou et al. 2018). In Klisova, only station 1 approached (species richness) or surpassed (Shannon–Wiener index) these values. Nevertheless, the total biodiversity of the lagoon was higher than these reference values. The only published study on the macrozoobenthos of the Klisova Lagoon dates back to 1983 (Bogdanos and Diapoulis 1984). These authors found a total of 28 species, several of which were also found in this study (e.g. Capitella capitata, Oxydromus pallidus (as Podarke pallida), Neanthes acuminata (as Neanthes caudata) and Naineris laevigata, Oligochaeta sp.). However, these authors used a larger mesh size of 1 mm and likely missed many of the meiofaunal-sized species found in this study, such as several species of Fabriciidae and Exogoninae. Furthermore, advances in taxonomy (such as revisions of taxa and descriptions of new species) as well as the different available identification literature make direct comparisons difficult.
According to the most recent River Basin Management Plans (RBMPs) compiled during the monitoring for the Water Framework Directive (2000/60/EC), the ecological status of the whole Messolonghi–Klisova lagoon complex has been characterised as ‘moderate’, taking into account various physicochemical and biological quality elements, and the chemical status has been characterised as ‘good’. Additionally, a ‘moderate’ ES was also found by Orfanidis et al. (2020) on the basis of the characteristics of C. nodosa. Here, we only assessed the ecological quality of the macrozoobenthos,which provided a rather positive assessment, as the macrobenthic communities at all but one station showed a good or high status., This positive classification could be owing to the fact that Western Klisova is practically isolated from the adjacent degrading pressures (e.g. irrigation waters, waste waters, etc.) which are responsible for various problems in other parts of the complex and have mainly been attributed to eutrophication (Gianni et al. 2013; Avramidis et al. 2017). Moreover, it has been suggested that the various biological quality elements used for the ES assessment present a higher ratio of disagreement in TWs compared with coastal environments (Borja and Rodriguez 2010) and as such, the implementation of the ‘one out–all out’ principle often leads to an underestimation of the actual ES of the system, a problem that has also been pointed out by Prato et al (2014).
The extensive presence of C. nodosa in Western Klisova can be considered as a significant indicator of ‘low pressure’ and good ecological status (however, no assessment sensu WFD has been done, in which additional parameters, such as phytoplankton, chlorophyll a (Chl-a), fish community etc., are taken into account). The presence of seagrasses in transitional waters has been linked to higher ecological status compared with unvegetated areas (Prato et al. 2014) and pristine coastal lagoons are in many cases dominated by extensive coverage of seagrass species, such as C. nodosa, Zostera noltei and Ruppia cirrhosa (Basset et al. 2006). Seagrasses reduce eutrophication, acting as water filters, transporting nutrients to the sediment and in parallel provide niche for invertebrates, fishes and macroalgae (Lloret and Marin 2009; Prato et al. 2014; Cabana et al. 2017), thus increasing the resilience of TWs to pressures. Owing to their ecological importance, detailed knowledge of the distribution of seagrasses in lagoons is an important prerequisite for successful management, and this study showed that remote sensing processing of Worldview-2 images allowed the exact mapping of C. nodosa and the detection of areas covered by bare sediments. Although ground truthing was performed approximately 1 year after the satellite image was taken, it did not seem to significantly affect our results. C. nodosa meadows can present stability and resilience in space and time (Peralta et al 2021) and large differences in their distribution are not expected in the time period of 1 year without any drastic parallel changes in the hydrological regime of the lagoon. As remote sensing presents the advantages of accuracy and repeatability, it could be a very useful way to detect the distribution of seagrass cover in similar lagoons and as a tool to monitor changes, thus allowing managerial bodies to take early conservation actions (Zoffoli et al. 2020).
C. nodosa in Klisova Lagoon during spring and summer, seems to be well acclimated to the hypersaline conditions in the lagoon, especially in the eastern part. This is also supported by the observed shoot density of the meadows which is within the reported density range (300–2000 shoots per m-2) in lagoons and estuarine ecosystems (Ferrat et al. 2003; Peralta et al. 2021). C. nodosa is the second most abundant seagrass species in the Mediterranean, colonising coastal and transitional waters as it is more tolerant to salinity fluctuations than Posidonia oceanica (Piro et al. 2015). Both field and experimental studies set its salinity tolerance limits between 20 and 54 and its optimal range for growth and survival in the range of 30–40 (Lloret et al. 2005; Fernandez-Torquemada and Sanchez-Lizaro 2011). Experimental studies in aquaria have demonstrated the negative effects of increased salinity in C. nodosa growth, survival, photosynthetic rate and metabolic processes (Garrote-Moreno et al. 2014; Pages et al. 2010; Piro et al. 2015; Tsioli et al. 2022) even after short periods of acclimation. However, it is possible that under natural conditions, in which salinity rises more gradually during the warm period, seagrass species have more time for acclimation and might present a higher tolerance to osmotic stress than in experimental conditions (Ralph 1998). In the present study, C. nodosa meadows were present in areas where measured salinity exceeded the reported tolerance limit of the species (i.e. 54). Whether this fact is owing to acclimation or tolerance of C. nodosa to these conditions, needs further investigation. It is possible that high salinity may have restricted the growth of the species only in one part of the lagoon (eastern part, around station 5) as this site coincided with the highest recorded salinity values. On the other hand, the absence of meadows in the bare area in the western part is human induced as it is caused by the use of this area for recreation, where hundreds of people use this part of the lagoon for swimming and mud baths during the whole summer.
This study provides a contribution to an unusual coastal ecosystem in Greece: a seasonally hypersaline lagoon with little biological/ecological zonation and high ecological value owing to its dense coverage by the seagrass C. nodosa. We acknowledge the limitations of the study, as not all parameters were measured at a high temporal frequency or over a long period of time. Regular seasonal sampling over several years would yield valuable additional insights into the complex interplay of the ecosystem components, but sampling strategies always need to be weighted against the available resources (Gray and Elliot 2009). We instead opted for denser spatial coverage, as recommended by Cabana et al. 2016 in their monitoring guidelines for Mediterranean lagoons, covering different habitats as well as the inner and outer part of the lagoon, and choosing a number of stations adequate to detect spatial gradients in the macrobenthic communities. This is in line with many other studies (e.g. Arvanitidis et al. 1999, Barbone et al. 2012, Bonifazi et al. 2023, Bray et al. 2022, Cabana et al. 2016, Giampaoletti et al. 2023, Pitacco et al. 2019, Reizopoulou et al. 2014, Simboura & Reizopoulou 2008). However, in a lagoon, such as Klisova, which is not monitored regularly under the WFD, even ‘snapshots’ of the conditions in the lagoon provide valuable information which can be used as a reference in the future. Our study demonstrates the value of an integrated approach in the ecological characterisation of various ecosystem components through different techniques: by combining remote sensing, ground truthing, in-situ analyses and geological and biological sample analysis a holistic image of the environmental conditions of the ecosystem could be constructed.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Arvanitidis C, Koutsoubas D, Dounas C, Eleftheriou A (1999) Annelid fauna of a Mediterranean lagoon (Gialova Lagoon, south-west Greece): community structure in a severely fluctuating environment. J Mar Biol Assoc UK 79:849–856. https://doi.org/10.1017/S0025315499001010
Avramidis P, Bekiari V (2021) Application of a catalytic oxidation method for the simultaneous determination of total organic carbon and total nitrogen in marine sediments and soils. PLoS ONE 16:e0252308. https://doi.org/10.1371/journal.pone.0252308
Avramidis P, Bekiari V, Tsiotsis E, Kalimani E (2010) Seasonal variation of nutrients, COD and BOD5in klisova lagoon channels (S.E. Mesolonghi Aetoliko Lagoon Complex)5 W. Greece Fresen Environ Bull 19:3242–3248
Avramidis P, Bekiari V, Christodoulou D, Papatheodorou G (2015) Sedimentology and water column stratification in a permanent anoxic Mediterranean lagoon environment, Aetoliko Lagoon, western Greece. Environ Earth Sci 73:5687–5701
Avramidis P, Nikolaou K, Poulos K, Bekiari V, Vantarakis A (2017) Environmental characterization of a Mediterranean protected shallow brackish coastal aquatic system, Klisova Lagoon, Western Greece: a case study. J Coast Conserv 21:115–125. https://doi.org/10.1007/s11852-016-0476-2
Barbone E, Rosati I, Reizopoulou S, Basset A (2012) Linking classification boundaries to sources of natural variability in transitional waters: a case study of benthic macroinvertebrates. Ecol Indic 12:105–122. https://doi.org/10.1016/j.ecolind.2011.04.014
Barnes RSK (2023) Seagrass macrobenthic biodiversity does not vary in conformity with a leaky-lagoonal confinement gradient. Mar Environ Res 185:105897. https://doi.org/10.1016/j.marenvres.2023.105897
Barnes RSK, Claassens L, Seath J (2022) Where ecologically ‘tis better to go brown than green: enhanced seagrass macrobenthic biodiversity within the canals of a brownfield coastal marina. Biodivers Conserv 31:2981–2997. https://doi.org/10.1007/s10531-022-02468-9
Basset A, Sabetta L, Fonnesu A, Mouillot D, Do Chi T, Viaroli P, Giordani G, Reizopoulou S, Abbiati M, Carrada GC (2006) Typology in Mediterranean transitional waters: new challenges and perspectives. Aquatic Conserv Mar Freshw Ecosyst 16:441–455. https://doi.org/10.1002/aqc.767
Basset A, Barbone E, Borja A, Brucet S, Pinna M, Quintana XD, Reizopoulou S, Rosati I, Simboura N (2012) A benthic macroinvertebrate size spectra index for implementing the water framework directive in coastal lagoons in Mediterranean and Black Sea ecoregions. Ecol Indic 12:72–83. https://doi.org/10.1016/j.ecolind.2011.06.012
Basset A, Elliott M, West RJ, Wilson JG (2013) Estuarine and lagoon biodiversity and their natural goods and services. Estuar Coast Shelf S 132:1–4. https://doi.org/10.1016/j.ecss.2013.05.018
Bird ECF (2011) Coastal geomorphology: an introduction. John Wiley & Sons, New York
Bogdanos C, Diapoulis A (1984) Contribution to the knowledge of the zoobenthos and phytobenthos of the Messolonghi lagoon. Thalassographica 7:39–52
Bologna PAX, Heck KL (2002) Impact of habitat edges on density and secondary production of seagrass-associated fauna. Estuaries 25:1033–1044. https://doi.org/10.1007/BF02691350
Bonifazi A, Galli S, Gravina MF, Ventura D (2023) Macrozoobenthos structure and dynamics in a Mediterranean hypersaline ecosystem with implications for wetland conservation. Water 15:1411. https://doi.org/10.3390/w15071411
Borja Á, Rodríguez JG (2010) Problems associated with the ‘one-out, all-out’ principle, when using multiple ecosystem components in assessing the ecological status of marine waters. Mar Pollut Bull 60:1143–1146. https://doi.org/10.1016/j.marpolbul.2010.06.026
Borja Á, Franco J, Pérez V (2000) A marine biotic index to establish the ecological quality of soft-bottom benthos within European Estuarine and coastal environments. Mar Pollut Bull 40:1100–1114
Boström C, Törnroos A, Bonsdorff E (2010) Invertebrate dispersal and habitat heterogeneity: expression of biological traits in a seagrass landscape. J Exp Mar Biol Ecol 390:106–117. https://doi.org/10.1016/j.jembe.2010.05.008
Boudouresque C, Bernard G, Pergent G, Shili A, Verlaque M (2009) Regression of Mediterranean seagrasses caused by natural processes and anthropogenic disturbances and stress: a critical review. Bot Mar. 52:395–418. https://doi.org/10.1515/BOT.2009.057
Bray JR, Curtis JT (1957) An ordination of the upland forest communities of Southern Wisconsin. Ecol Monogr 27:325–349
Bray L, Faulwetter S, Kaberi H, Karageorgis AP, Kastanidi E, Katsiaras N, Pavlidou A, Providakis N, Sigala K, Voutsinas E, Zeri C, Reizopoulou S (2022) Assessing pressure drivers on the benthic ecosystem in the coastal zone of Western Messinia, Greece. Estuar Coast Shelf S 274:107935. https://doi.org/10.1016/j.ecss.2022.107935
Cabana D, Sigala K, Nicolaidou A, Reizopoulou S (2013) Towards the implementation of the water framework directive in Mediterranean transitional waters: the use of macroinvertebrates as biological quality elements. Adv Oceanogr Limnol 4:212–240. https://doi.org/10.1080/19475721.2013.846279
Cabana D, Nicolaidou A, Sigala K, Reizopoulou S (2017) Multi-scale functional and taxonomic β-diversity of the macroinvertebrate communities in a Mediterranean coastal lagoon. Mediterr Mar Sci. 18:121. https://doi.org/10.12681/mms.1844
Cabana D, Sigala K, Nicolaidou A, Reizopoulou S (2016) Monitoring guidelines for Mediterranean coastal lagoons. The use of benthic macroinvertebrate communities as biological quality indicators. In: MMMPA Supervisory Board (Eds) Monitoring Mediterranean Marine Protected Areas: a set of guidelines to support the development of management plans. Deliverable of the MMMPA European project (FP7-PEOPLE-2011-ITN g.a. no.: 290056). Ancona, 116 pages. pp 63–69-xx
Chatzikakidis AD (1953) Seasonal hydrological studies in the lagoons of Messolongi and Aitoliko. Proceedings of Greek Hydrobiological Institute of Athens Academy Vol VI. http://repository.library.teimes.gr/xmlui/handle/123456789/2204. Accessed 12 Jan 2024
Christia C, Papastergiadou E (2007) Spatial and temporal variations of aquatic macrophytes and water quality in six coastal lagoons of western Greece. Belg J Bot 140:39–50
Christia C, Giordani G, Papastergiadou E (2018) Environmental variability and macrophyte assemblages in coastal lagoon types of Western Greece (Mediterranean Sea). Water 10:151
Connolly RM (1997) Differences in composition of small, motile invertebrate assemblages from seagrass and unvegetated habitats in a southern Australian estuary
Dauvin JC, Ruellet T (2007) Polychaete/amphipod ratio revisited. Mar Pollut Bull 55:215–224. https://doi.org/10.1016/j.marpolbul.2006.08.045
Edgar GJ (1994) Comparisons of species richness, size-structure and production of benthos in vegetated and unvegetated habitats in Western Fort, Victoria. J Exp Mar Biol Ecol 176:201–226
Emmanouilidis A, Panagiotopoulos K, Kouli K, Avramidis P (2022) Late-Holocene paleoenvironmental and land-use changes in Western Greece based on a sediment record from Klisova lagoon. Holocene 32(6):485–500
Fahrig L (2003) Effects of habitat fragmentation on biodiversity. Annu Rev Ecol Evol 34:487–515. https://doi.org/10.1146/annurev.ecolsys.34.011802.132419
Faulwetter S, Simboura N, Katsiaras N, Chatzigeorgiou G, Arvanitidis C (2017) Polychaetes of Greece: an updated and annotated checklist. Biodiversity Data Journal 5:e20997. https://doi.org/10.3897/BDJ.5.e20997
Fernández-Torquemada Y, Sánchez-Lizaso JL (2011) Responses of two Mediterranean seagrasses to experimental changes in salinity. Hydrobiologia 669:21–33. https://doi.org/10.1007/s10750-011-0644-1
Ferrat L, Fernandez C, Pasqualini V, Pergent G, Pergent-Martini C (2003) Evolution and vitality of seagrasses in a Mediterranean lagoon. J Environ Sci Health 8:1459–1468
Folk RL (1974) Petrology of sedimentary rocks. Hemphill Publishing Co., Austin
Garrote-Moreno A, Fernández-Torquemada Y, Sánchez-Lizaso JL (2014) Salinity fluctuation of the brine discharge affects growth and survival of the seagrass Cymodocea nodosa. Mar Pollut Bull 81:61–68. https://doi.org/10.1016/j.marpolbul.2014.02.019
Gaudette H, Flight W, Toner L, Folger D (1974) An inexpensive titration method for the determination of organic carbon in recent sediments. J Sediment Petrol 44:249–253
Giampaoletti J, Sbrana A, Magni P, Gravina MF (2023) Macrobenthos of the Tortolì Lagoon: a peculiar case of high benthic biodiversity among Mediterranean lagoons. Diversity 15:783. https://doi.org/10.3390/d15060783
Gianni A, Zacharias I (2012) Modeling the hydrodynamic interactions of deep anoxic lagoons with their source basins. Estuar Coast Shelf S 110:157–167. https://doi.org/10.1016/j.ecss.2012.04.030
Gianni A, Zamparas M, Papadas IT, Kehayias G, Deligiannakis Y, Zacharias I (2013) Monitoring and modeling of metal concentration distributions in anoxic basins: Aitoliko Lagoon, Greece. Aquat Geochem 19:77–95. https://doi.org/10.1007/s10498-012-9179-y
Gray JS, Elliott M (2009) The ecology of marine sediments: from science to management, 2nd edn. Oxford University Press, Oxford, New York, p 225
Guelorget O, Perthuisot JP (1983) Le domaine paralique: expressions géologiques, biologiques et économiques du confinement. Travaux Du Laboratoire De Géologie, Ecole Normale Superiere Paris 16:1–136
Hotos GN, Avramidou DE (1997) A one year water monitoring study of Klisova lagoon (Mesolonghi, W. Greece). GeoJournal 41:15–23. https://doi.org/10.1023/A:1006805515055
Jones GA, Kaiteris P (1983) A vacuum-gasometric technique for rapid and precise analysis of calcium carbonate in sediments and soils. J Sediment Res 53:655–660. https://doi.org/10.1306/212F825B-2B24-11D7-8648000102C1865D
Karageorgis AP, Sioulas A, Krasakopoulou E, Anagnostou CL, Hatiris GA, Kyriakidou H, Vasilopoulos K (2012) Geochemistry of surface sediments and heavy metal contamination assessment: Messolonghi lagoon complex, Greece. Environ Earth Sci 65:1619–1629. https://doi.org/10.1007/s12665-011-1136-3
Katselis G, Koutsikopoulos C, Dimitriou E, Rogdakis Y (2003) Spatial patterns and temporal trends in the fisheries landings of the Messolonghi-Etoliko lagoons (Western Greek Coast). Sci Mar. 67:501–511. https://doi.org/10.3989/scimar.2003.67n4501
Kjerfve B (1986) Comparative oceanography of coastal lagoons. In: Wolfe DA (ed) Estuarine variability. Academic Press, New York, pp 63–81. https://doi.org/10.1016/B978-0-12-761890-6.50009-5
Kjerfve B (1994) Coastal lagoons. In: Kjerfve B (ed) Coastal lagoon processes. Elsevier Oceanographic Series, Amsterdam, pp 1–8
Kyriou A, Nikolakopoulos K (2020) Landslide mapping using optical and radar data: a case study from Aminteo, Western Macedonia Greece. Europe J Remote Sens 53:17–27. https://doi.org/10.1080/22797254.2019.1681905
Lloret J, Marín A (2009) The role of benthic macrophytes and their associated macroinvertebrate community in coastal lagoon resistance to eutrophication. Mar Pollut Bull 58:1827–1834. https://doi.org/10.1016/j.marpolbul.2009.08.001
Lloret J, Marin A, Marin-Guirao L, Velasco J (2005) Changes in macrophytes distribution in a hypersaline coastal lagoon associated with the development of intensively irrigated agriculture. Ocean Coast Manage 48:828–842. https://doi.org/10.1016/j.ocecoaman.2005.07.002
Magni P, Vesal SE, Giampaoletti J, Como S, Gravina MF (2023) Joint use of biological traits, diversity and biotic indices to assess the ecological quality status of a Mediterranean transitional system. Ecol Indic 147:109939. https://doi.org/10.1016/j.ecolind.2023.109939
Marazioti CE, Flessia G, Vlachos P, Koutrouli E, Ntaikou I, Antonopoulou G, Pakou C, Kalfas C, Kornaros M, Lyberatos G (2010) On the environmental state of the Mesologgi lagoon in Greece. Fresen Environ Bull 19:1151–1164
Moore EC, Hovel KA (2010) Relative influence of habitat complexity and proximity to patch edges on seagrass epifaunal communities. Oikos 119:1299–1311. https://doi.org/10.1111/j.1600-0706.2009.17909.x
Müller G, Gastner M (1971) The “Karbonat-Bombe”, a simple device for the determination of carbonate content in sediment, soils, and other materials. In: Neues Jahrbuch für Mineralogie—Monatshefte. https://epic.awi.de/id/eprint/27239/. Accessed 26 Jan 2023
Muxika I, Borja Á, Bald J (2007) Using historical data, expert judgement and multivariate analysis in assessing reference conditions and benthic ecological status, according to the European Water Framework Directive. Mar Pollut Bull 55:16–29. https://doi.org/10.1016/j.marpolbul.2006.05.025
Nichols M, Allen G (1981) Sedimentary processes in coastal lagoons. In: UNESCO Technical Papers in Marine Science (UNESCO). UNESCO, Div. of Marine Sciences.
Nicolaidou A, Reizopoulou S, Koutsoubas D, Orfanidis S, Kevrekidis T (2005) Biological components of Greek lagoonal ecosystems: an overview. Mediterr Mar Sci. 6:31–50. https://doi.org/10.12681/mms.184
Nikolakopoulos KG (2008) Comparison of nine fusion techniques for very high resolution data. Photogramm Eng Rem S. 74:647–659. https://doi.org/10.14358/PERS.74.5.647
Nikolakopoulos K, Oikonomidis D (2015) Quality assessment of ten fusion techniques applied on Worldview-2. Eur J Remote Sens 48:141–167. https://doi.org/10.5721/EuJRS20154809
Oksanen J, Blanchet FG, Friendly M, Kindt R, Legendre P, McGlinn D, Minchin PR, O’Hara RB, Simpson GL, Solymos P, Henry M, Stevens H, Szoecs E, Wagner H (2020) vegan: community ecology package. R package version 2.5–7. https://CRAN.R-project.org/package=vegan
Orfanidis S, Papathanasiou V, Mittas N, Theodosiou T, Ramfos A, Tsioli S, Kosmidou M, Kafas A, Mystikou A, Papadimitriou A (2020) Further improvement, validation, and application of CymoSkew biotic index for the ecological status assessment of the Greek coastal and transitional waters. Ecol Indic 118:106727. https://doi.org/10.1016/j.ecolind.2020.106727
Pagès JF, Pérez M, Romero J (2010) Sensitivity of the seagrass Cymodocea nodosa to hypersaline conditions: a microcosm approach. J Exp Mar Biol Ecol 386:34–38. https://doi.org/10.1016/j.jembe.2010.02.017
Papatheodorou G, Hotos G, Geraga M, Avramidou D, Vorinakis T (2002) Heavy metal concentrations in sediments of Klisova lagoon (southeast Mesolonghi-Aetolikon lagoon complex). W Greece Fresen Environ Bull 11:951–956
Peralta G, Godoy O, Egea LG, De Los Santos CB, Jiménez-Ramos R, Lara M, Brun FG, Hernández I, Olivé I, Vergara JJ, González-Ortiz V, Moreno-Marín F, Morris EP, Villazán B, Pérez-Lloréns JL (2021) The morphometric acclimation to depth explains the long-term resilience of the seagrass Cymodocea nodosa in a shallow tidal lagoon. J Environ Manag 299:113452. https://doi.org/10.1016/j.jenvman.2021.113452
Pergent G, Djellouli A, Hamza AA, Ettayeb KS, El Mansouri AA, Talha FM, Hamza MA, Pergent-Martini C, Platini F (2002) Characterization of the benthic vegetation in the Farwà Lagoon (Libya). J Coast Conserv 8:119. https://doi.org/10.1652/1400-0350(2002)008[0119:COTBVI]2.0.CO;2
Phleger FB (1981) A review of some general features of coastal lagoons. UNESCO technical papers in marine science. Coastal Lagoon Research, past, Present and Future 33:7–14
Pierre St. JI, Kovalenko KE (2014) Effect of habitat complexity attributes on species richness. Ecosphere 5:22. https://doi.org/10.1890/ES13-00323.1
Piro A, Marín-Guirao L, Serra IA, Spadafora A, Sandoval-Gil JM, Bernardeau-Esteller J, Fernandez JMR, Mazzuca S (2015) The modulation of leaf metabolism plays a role in salt tolerance of Cymodocea nodosa exposed to hypersaline stress in mesocosms. Front Plant Sci 6:464. https://doi.org/10.3389/fpls.2015.00464
Pitacco V, Reizopoulou S, Sfriso A, Sfriso A, Mistri M, Munari C (2019) The difficulty of disentangling natural from anthropogenic forcing factors makes the evaluation of ecological quality problematic: a case study from Adriatic lagoons. Mar Env Res 150:104756. https://doi.org/10.1016/j.marenvres.2019.104756
Prato S, La Valle P, De Luca E, Lattanzi L, Migliore G, Morgana JG, Munari C, Nicoletti L, Izzo G, Mistri M (2014) The “one-out, all-out” principle entails the risk of imposing unnecessary restoration costs: a study case in two Mediterranean coastal lakes. Mar Pollut Bull 80:30–40. https://doi.org/10.1016/j.marpolbul.2014.01.054
Ralph PJ (1998) Photosynthetic responses of Halophila ovalis (R. Br.) Hook. f. to osmotic stress. J Exp Mar Biol Ecol 227:203–220. https://doi.org/10.1016/S0022-0981(97)00269-4
Ramfos A, Somarakis S, Koutsikopoulos C, Fragopoulu N (2005) Summer mesozooplankton distribution in coastal waters of central Greece (eastern Mediterranean). I. Hydrology and group composition. J Mar Biol Assoc UK 85:755–764. https://doi.org/10.1017/S0025315405011665
Reizopolou S, Penna M, Boix D, Buchet R, Costas N, Derolez V, Gascon S, Gifre J, Martinoy M, Pardo I, Quintana X, Trabucco B, Sala J, Salas Herrero F (2018) Transitional waters Mediterranean geographic intercalibration group: benthic invertebrates fauna ecological assessment methods. Publications Office of the European Union, LU Available from: https://op.europa.eu/en/publication-detail/-/publication/1dbb5a08-0fdc-11e9-81b4-01aa75ed71a1/language-en/format-PDF/source-278710104 (January 13, 2023)
Reizopoulou S, Nicolaidou A (2004) Benthic diversity of coastal brackish-water lagoons in western Greece. Aquatic Conserv 14:S93–S102. https://doi.org/10.1002/aqc.653
Reizopoulou S, Nicolaidou A (2007) Index of size distribution (ISD): a method of quality assessment for coastal lagoons. Hydrobiologia 577:141–149. https://doi.org/10.1007/s10750-006-0423-6
Reizopoulou S, Kormas KA, Nicolaidou A (1998) Benthic biodiversity in five coastal brackish water lagoons of Amvrakikos Gulf, Hellas. Rapports De Committee Int De Mer Mediterranee 35:580–581
Reizopoulou S, Simboura N, Barbone E, Aleffi F, Basset A, Nicolaidou A (2014) Biodiversity in transitional waters: steeper ecotone, lower diversity. Mar Ecol 35:78–84. https://doi.org/10.1111/maec.12121
Shannon CE (1948) A mathematical theory of communication. Bell Syst Tech J. 27:379–423
Signorini A, Massini G, Migliore G, Tosoni M, Varrone C, Izzo G (2008) Sediment biogeochemical differences in two pristine Mediterranean coastal lagoons (in Italy) characterized by different phanerogam dominance-a comparative approach. Aquatic Conserv Mar Freshw Ecosyst 18:S27–S44. https://doi.org/10.1002/aqc.953
Simboura N, Reizopoulou S (2008) An intercalibration of classification metrics of benthic macroinvertebrates in coastal and transitional ecosystems of the Eastern Mediterranean ecoregion (Greece). Mar Pollut Bull 56:116–126. https://doi.org/10.1016/j.marpolbul.2007.09.042
Simboura N, Zenetos A (2002) Benthic indicators to use in ecological quality classification of Mediterranean soft bottom marine ecosystems, including a new Biotic Index. Mediterr Mar Sci 3:77–111
Tanner JE (2005) Edge effects on fauna in fragmented seagrass meadows. Austral Ecol 30:210–218. https://doi.org/10.1111/j.1442-9993.2005.01438.x
Tsioli S, Koutalianou M, Gkafas GA, Exadactylos A, Papathanasiou V, Katsaros CI, Orfanidis S, Küpper FC (2022) Responses of the Mediterranean seagrass Cymodocea nodosa to combined temperature and salinity stress at the ionomic, transcriptomic, ultrastructural and photosynthetic levels. Mar Environ Res 175:105512. https://doi.org/10.1016/j.marenvres.2021.105512
Urra J, Mateo Ramírez Á, Marina P, Salas C, Gofas S, Rueda JL (2013) Highly diverse molluscan assemblages of Posidonia oceanica meadows in northwestern Alboran Sea (W Mediterranean): seasonal dynamics and environmental drivers. Estuar Coast Shelf S 117:136–147. https://doi.org/10.1016/j.ecss.2012.11.005
Verdonschot RCM, Didderen K, Verdonschot PFM (2012) Importance of habitat structure as a determinant of the taxonomic and functional composition of lentic macroinvertebrate assemblages. Limnologica 42:31–42. https://doi.org/10.1016/j.limno.2011.07.004
WoRMS Editorial Board (2024) World register of marine species. https://www.marinespecies.org at VLIZ. Accessed 03 Nov 2024
Zavodnik N, Travizi A, De Rosa S (1998) Seasonal variations in the rate of pliotosynthetic activity and chemical composition of the seagrass Cymodocea nodosa (Ucr.) Asch. Sci Mar. 62:301–309
Zoffoli ML, Gernez P, Rosa P, Le Bris A, Brando VE, Barillé A-L, Harin N, Peters S, Poser K, Spaias L, Peralta G, Barillé L (2020) Sentinel-2 remote sensing of Zostera noltei-dominated intertidal seagrass meadows. Remote Sens Environ 251:112020. https://doi.org/10.1016/j.rse.2020.112020
Acknowledgements
The authors would like to thank the Fishermen association of Klisova lagoon for their assistance in sampling. Likewise, Dr. Alexandros Emmanouilidis is acknowledged for his help in field work and Dr. Nikos Vlachos for his valuable help during the sorting of the organisms in benthic samples.
Funding
Open access funding provided by HEAL-Link Greece. The authors did not receive financial support for the submitted work. All authors certify that they have no affiliations with or involvement in any organisation or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
Author information
Authors and Affiliations
Contributions
P.A and A.R conceptualised the study; P.A, A.R, S.F and K.N performed the methodology; A.R, S.F and K.N conducted formal analysis and investigation; A.R, S.F, P.A and K.N performed writing – original draft preparation and writing – review and editing; P.A and A.R. provided the necessary resources; P.A supervised the study.
Corresponding author
Ethics declarations
Competing interests
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
Ethical approval
No approval of research ethics committees was required to accomplish the goals of this study because work was conducted with unregulated invertebrate species.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ramfos, A., Alysandratou, A., Katsani, O. et al. Ecological and environmental characteristics of a seagrass-dominated hypersaline coastal Mediterranean lagoon: a multidisciplinary approach. Aquat Sci 86, 48 (2024). https://doi.org/10.1007/s00027-024-01063-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00027-024-01063-0