Abstract
At the start of the 21st century, a coastal residential-estate marina was developed on a previously degraded and polluted brownfield island site within Knysna estuarine bay, Garden Route National Park, South Africa, including the creation of 25 ha of new flow-through tidal canals. Canals near the larger entrance to this system now support permanently submerged beds of seagrass, which in turn support abundant macrobenthic invertebrates. In comparison with equivalent seagrass-associated assemblages present in natural channels around the island, those in the artificial marina canals were similarly structured and dominated by the same species, but the marina assemblages were significantly more species-rich (1.4 x on average) and were more abundant. Indeed, this area of marina supports the richest seagrass-associated macrofaunal biodiversity yet recorded from South Africa. The canals created de novo therefore now form a valuable addition to the bay’s marine habitat, in marked contrast to the generality that marinas developed on greenfield sites represent a net reduction in intertidal and shallow marine area and associated seagrass-associated benthos. If located and constructed appropriately, brownfield marina development and conservation of coastal marine biodiversity clearly need not be antithetical, and brownfield sites may provide opportunity for the location and management of ‘artificial marine micro-reserves’ or for the action of ‘other effective area-based conservation measures’ for soft-sediment faunas.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Marine coastal systems are becoming increasingly urbanised (Airoldi et al. 2008; Bishop et al. 2017), and this development includes the many residential marina estates that have appeared along sheltered stretches of the world’s coastline over the last few decades (Waltham and Connolly 2011). Indeed “there is barely a waterfront city or community in the developed world that doesn’t have a marina … [and] entire self-contained cities … [have] been developed around a new harbour and marina complex” (Natchez 2018). Many more are proposed, several in areas of shore and shallow sea notionally protected by Ramsar, Marine Park or equivalent conservation declarations (Sheppard 2019). It is estimated that between 1984 and 2016 16% of the world’s intertidal-flat habitat was lost to a range of causes, predominant among which was coastal development (Murray et al. 2019, 2022).
Although the negative effects of marina construction, especially on shorebirds (e.g. Duggan et al. 2011; Bishop et al. 2017), the spread of non-native species (Bulleri and Chapman 2010; Foster et al. 2016), and water quality (Rivero et al. 2013; Valdor et al. 2019), have caused widespread conservation concern, research on the endangered estuarine seahorse, Hippocampus capensis, has shown that this species has benefitted from the suitable new subtidal habitat provided by marina canals (Claassens 2016; Claassens and Hodgson 2018). A positive effect of marinas on the survival of juvenile native seabreams, Diplodus spp, along the French Mediterranean coast has also been suggested (Bouchoucha et al. 2016), although this may be an artifact of acting as attractors of juvenile fish (Bosch et al. 2017). The impact of coastal marinas has generated a large volume of research but relatively little of this has concerned their effect on soft-sediment benthic invertebrates and effectively none on seagrass macrobenthos or on the new aquatic habitats constructed on brownfield sites (i.e. on previously developed land no longer in use); although unsurprisingly it is known that hard surfaces are a ‘poor substitute’ for natural soft-sediment habitats including seagrass beds (Momota and Hosokawa 2021).
Thesen Islands Marina is one such 96 ha artificial residential-estate complex located within the marine embayment section of the warm-temperate Knysna estuarine bay, part of South Africa’s Garden Route National Park. It was constructed on a brownfield terrestrial site in the early 21st century (2000–2007) and includes some 25 ha of flow-through tidal canals, dug de novo across a former bay island and lined by gabions and floored by Reno mattress® (Claassens 2016). As Waltham and Connolly (2011) emphasised, research on the value of marina-associated waterways as aquatic habitats can and should underpin planning and management of these systems. Therefore to address this lack, the present study extended earlier work carried out on the macrobenthic invertebrate biodiversity associated with subtidal seagrass along the axial channel at Knysna (Barnes and Claassens 2020) by comparing this natural Zostera capensis system to that associated with the same species in the adjacent, recently-developed area of the artificial Thesen marina-canal system. The study examined the hypothesis that the benthic invertebrate fauna supported by the Zostera in the artificial marina-canals is an impoverished version of that present in natural beds of the same seagrass in the channels surrounding the marina, both in terms of abundance and biodiversity.
Methods
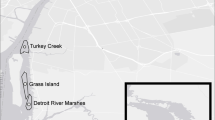
Macrofaunal sampling in the Thesen canals was conducted by snorkelling mainly during the 2020 austral summer, the research being approved by SANParks and conducted in accordance with their scientific research regulations and requirements. Two sites, c. 100 m apart, near the western entrance known to support Zostera (Claassens 2016) (at 34º02’58.1"S.23º02’51.7"E & 34º02’59.3"S.23º02’57.7"E, Fig. 1) were sampled at a depth of some 2 m below LWS using identical procedures to those described earlier for the natural subtidal sites along the Knysna axial channel (Barnes and Claassens 2020), i.e. each site by 16 cores each of 0.0054 m2 area and 100 mm depth. For one of the two sites, the onset of covid-19 related restrictions on travel and fieldwork prevented completion of sample collection in 2020, and the remaining cores were taken in summer 2022: species density values were lower in 2022 than in 2020 although the difference was not significant (ANOVA F1,14 = 1.9; P = 0.08).
Knysna estuarine bay (Google Earth image), showing the location of sampling sites in the natural Zostera-floored channels near Thesen Island (‘Brenton’ and ‘Steenbok’) and those in the artificial canals (‘Thesen’) of the Thesen Islands residential marina estate (location of the canal sites shown in greater detail in box)
Cores were gently sieved (‘puddled’) through 710 µm mesh on site. This sampling procedure collects the smaller and more numerous members of the macrofauna that constitute the large majority of invertebrate biodiversity (Bouchet et al. 2002; Albano et al. 2011), though not the meiofauna nor much scarcer megafauna nor sessile animals attached to the seagrass leaves. Warwick et al. (2006) have shown that different patterning rules may apply to meiofauna and macrofauna, and likewise Davidson et al. (2004) and Leopardas et al. (2014) to sessile species. Sessile or semi-sessile species such as Siphonaria compressa and Anomia achaeus that had accidentally become detached from the seagrass leaves during sampling were therefore ignored. Animals were identified to species level wherever possible, with all organismal nomenclature being as listed in the World Register of Marine Species (www.marinespecies.org) accessed December 2021, except in respect of the unlisted ‘Assiminea’ capensis (see Barnes 2018). It should be noted, however, that the specific identity of several animals, especially amongst the Polychaeta, is questionable because of lack of recent revision; those of South African taxa of Polycladida, Oligochaeta and Nemertini, and many members of other groups less than 3–4 mm in largest dimension, are virtually unknown. Such animals were treated as morphospecies, an operationally appropriate procedure to detect spatial patterns of numbers of species and their differential abundance (Dethier and Schoch 2006; Gerwing et al. 2020).
Analyses
Macrobenthic assemblage data from the marina-canal Zostera were compared with the equivalent relevant data for the macrobenthic assemblages in natural subtidal channels obtained by Barnes and Claassens (2020) in the same summer of 2020, i.e. those located at the nearby Brenton and Steenbok localities at similar depth below LWS (Fig. 1). The Steenbok locality was in the southern mouth of the Ashmead Channel that separates Thesen Island from both the mainland and from the nearby Leisure Isle; it was floored by clean tidal-delta sand and subject to strong water flow. That at Brenton was more sheltered and was floored by muddy sand. All three localities are fully marine and bathed by semidiurnal fluxes of water from the Indian Ocean. Numbers of each component zoobenthic taxon at the natural and artificial seagrass sites were subjected to similarity analysis, and assemblage metrics per unit area were derived and compared via PAST 4.0.9 software (Hammer et al. 2019), all based on abundance data (Beck et al. 2013).
Univariate metrics assessed were those known to have a major influence on local-scale biodiversity patterns (Blowes et al. 2022); i.e. (i) overall faunal numbers per unit area, (ii) observed numbers of taxa per unit area, N0 [i.e. ‘species density’ sensu (Gotelli and Colwell 2001)], and (iii) relative evenness (= equitability) assessed as Pielou’s J. Estimates of likely total number of taxa per unit area were obtained via Chao1 and ACE (abundance-based coverage estimation) values. In addition, and as previously at Knysna (Barnes 2019), patchiness in spatial abundance of the macrofaunal associations at each site and station was also quantified by spatial point pattern analysis using Lloyd’s (1967) index of patchiness, Ip. Multivariate comparison of macrofaunal assemblage composition used hierarchical clustering analysis of S17 Bray-Curtis similarity (Legendre and Legendre 1998), ANOSIM, PERMANOVA and SIMPER, carried out with 9999 permutations. Differentials between the structure of artificial marina-canal and natural-channel assemblages were also assessed by comparison of ranked taxon index of numerical importance (INI) distributions, analogous to species abundance distributions (Antão et al. 2021) but derived from both abundance and occupancy data (Barnes 2014). For this, all data sets were standardised for overall taxon density (by dividing all ranks by the total number of taxa in the set) and for sample size (by dividing the abundance of each taxon by the overall number of individuals in the set) (Passy 2016). Curves were compared using one-way ANCOVA, and differences in number of taxa and of individuals per core between the three localities were tested by one-way ANOVA and post hoc Tukey HSD tests.
Results
In total, the 96 subtidal samples yielded 16,407 animals of 116 taxa at an overall density of 31,500 m− 2, of which gastropod molluscs comprised 88% and the microphytobenthically-feeding cerithioid microgastropod Alaba pinnae 82%. The 32 samples from the marina canals totalled 6,870 individuals of 83 taxa from a total area of 0.17 m2; a local density of 39,757 m2 — these values constitute the most biodiverse fauna of any South African Zostera bed, and estimates of the true number of taxa in the sampled area at Thesen are considerably larger still at Chao1 = 129 and ACE = 121, one third of the taxa being singletons. The 64 natural-channel samples yielded a total of 86 taxa and a smaller estimates of likely total numbers, i.e. Chao1 = 109 and ACE = 108, with similar proportions of singletons (30–34%). Even though only formed less than 20 years ago, the marina Zostera beds therefore supported more macrobenthic species per unit area than either of the two natural channels investigated (1.38 x as many per core sample on average) (ANOVA F2,93 = 29; Tukey HSD P < < 0.0001) (Table 1). Core samples from the marina canals also yielded 1.44 x as many individual animals as the average for the natural channel sites, although this difference was (marginally) not significant (ANOVA F2,93 = 2.87; P = 0.06). The difference in numbers of non-Alaba individuals, however, was highly significant (ANOVA F2,93 = 22; P < < 0.0001; all Tukey HSD P < 0.02); whereas those in Alaba numbers was not (ANOVA F2,93 = 1.2; P = 0.3).
The seagrass-associated species concerned were mostly classic members of the southern African estuarine fauna as identified by Day (1981) and de Villiers et al. (1999), but the superabundance of the Zostera-leaf associated Alaba pinnae appears most unusual. Alaba is also present, though by no means dominant, in the nearby Swartvlei estuary to the west (Whitfield 1989) but has not been recorded by surveys of other South African estuaries (Barnes and Claassens 2020), except sporadically in the St Lucia / iSimangaliso system (Perissinotto et al. 2014), and apparently it does not occur in the Keurbooms/Bitou Estuary immediately to the east of Knysna (Duvenage and Morant 1984; Villiers et al. 2021). Leaving aside the overwhelmingly dominant Alaba, with the exception of a single core sample containing large numbers of small nereid worms, compared to the natural channels the Thesen canals supported proportionately fewer polychaete individuals (11% of the total vs. 20%) and those of other gastropod taxa (28% vs. 37%), but more crustaceans (48% vs. 37%), bivalves (5% vs. 2%) and echinoderms (3% vs. 1%) (see Supplementary data). These relative abundances of polychaetes, crustaceans and echinoderms are the same as those of the MLW → LWS → subtidal gradient at Brenton and Steenbok (from data of Barnes and Claassens 2020), although this may be purely coincidental.
Excluding rarities, no species common to the two natural channels was noticeably absent from the Thesen canals, nor was any species present there not also represented in the natural channels, except the small bivalve Gafrarium pectinatum which occurred in the canals at a density of 214 m− 2, although the amphipods Ericthonius and Monocorophium, and the gastropod Gibbula were at least six times more abundant in the Zostera of the canals than outside the marina. The two amphipods Ericthonius and Monocorophium are certainly aliens or cryptogenics (Griffiths et al. 2009), as are the amphipod Jassa and isopod Paracerceis: all four are now distributed right across the globe [Global Biodiversity Information Facility (www.gbif.org), accessed February 2022]. Erichthonius and Jassa were present in Knysna before 1950 (Day et al. 1951), whilst the other two are perhaps more recent arrivals, although they also predate the Thesen marina. Both are widespread along the South African coast having been distributed in association with oyster cultivation and via commercial shipping (Griffiths et al. 2011), two activities that have influenced the Knysna marina fauna for many decades.
Other notable occurrences in the canals included the rare and critically endangered, seagrass false-limpet Siphonaria compressa and the uncommon seagrass-associated form of the starfish Parvulastra exigua — both known only from two localities, both in the Western Cape (Knysna and the Langebaan estuarine lagoon). An Elysia amongst the collected material is almost certainly a new species (T. Gosliner pers com). The suites of numerically dominant species at the three localities are shown in Table 2.
Variation in the abundance of Alaba accounted for > 65% of the assemblage compositional differences between the three localities (SIMPER); all comparisons between individual sites yielding significant differences (ANOSIM R ≥0.12; P < 0.02; PERMANOVA F > 4; P < 0.008) except that between the two Brenton sites. At the level of the three localities, all three were significantly different (PERMANOVA F > 4.8; P < 0.003). Bray-Curtis similarities between the macrobenthic assemblages (Fig. 2) indicated that the artificial Thesen sites were nested within those of the two local natural channels; that between the natural- and artificial-channel assemblages being 0.73. The greater abundance of Alaba, Gibbula, Ericthonius and ?Cylindroleberis, and the smaller densities of ‘Assiminea’, Turritella and Grandidierella, in the Thesen canals accounted for > 80% of such differences between the artificial canals and natural channels as there were (SIMPER). To a large extent Grandidierella was replaced in the canals by another aorid ?Bemlos sp. that was more than twice as abundant there as in the natural channels.
Assemblages values of evenness in the artificial canals were within the natural-channel range, and there was no significant difference between the ranked index of numerical importance curves for the assemblages in the natural channels and the artificial canals (ANCOVA F = 0.02; Psame = 0.89 for both mean values and slopes; Fig. 3). Values of patchiness were all considerably greater and more variable than those noted earlier for Knysna intertidal seagrass macrobenthic assemblages (Barnes 2019), mainly consequent on the large range in density of Alaba (2-868 per core; mean 140 ± 15 S.E.; Ip = 1.8–2.3; P < 0.0001).
In summary, the area within the larger entrance to the artificial marina-canal system of the Thesen Islands township, an area set aside and approved for development, therefore supported a greater abundance and greater biodiversity of seagrass-associated macrobenthos than did the Zostera in the natural channels of the Knysna marine embayment surrounding it, i.e. in those regions conserved as a Protected Environment under the South African National Environmental Management Protected Areas Act (No. 57 of 2003). Thus the tested hypothesis was disproved.
Discussion
One of the known major influences of marinas, as of coastal urbanisation in general, is the addition of abundant hard surfaces to which sessile marine species can attach (Mineur et al. 2012; Rivero et al. 2013; Momota and Hosokawa 2021), including unwanted aliens. They also might be expected to impinge, however, on the ecology of areas of soft sediment, although from what little information appears to be available, it would seem that the benthic macrofauna of subtidal bare sediment is least affected by their presence. Thus Baird et al. (1981), in respect of an estuarine marina in the Eastern Cape, South Africa, and Chou et al. (2015), for three marinas in different stretches of Singapore’s coast, both found minimal consistent difference in faunal abundance and biodiversity inside and outside the marinas concerned, although the Singapore marina subject to least water flow experienced marked siltation and supported a reduced fauna dominated by a few opportunistic cirratulid and spionid polychaetes (see Maxted et al. 1997). Nevertheless, high levels of domestic and other pollutants can accumulate within the sediments of marina complexes (Guerra-García et al. 2021), as in other harbours (Guerra-García and García-Gómez 2005), and these may have consequences on faunal abundance and composition. Macrofaunas of areas covered by seagrass, however, are greatly affected; coastal development, including marina estates, being regarded as a major destroyer of seagrass beds and their inhabitants (Duarte et al. 2004).
Although many beds have been lost to concrete, there is evidence that seagrass systems can survive in close proximity to coastal development (Blake et al. 2014). At Knysna, this clearly includes very close to developed areas. The rich, Alaba-dominated, subtidal benthic macrofauna associated with Zostera capensis in the marine embayment section of the Knysna estuarine bay continues into the canal system of the brownfield Thesen marina with some increase in abundance of Alaba but especially with a significant increase in both abundance and species-richness of the associated fauna. Not all the canal system appears suitable for seagrass, however; indeed Zostera is present over less than 2% of the total area of marina waterways (Claassens 2016), being largely restricted to the high water-velocity region adjacent to the western entrance to the system. Areas with less current velocity support Codium tenue or mixed algal and seagrass communities (Asparagopsis, Caulerpa, Halodule, etc.) instead (Claassens 2016). But where it is present it grows luxuriously (leaf length > 1 m) and clearly supports a flourishing macrobenthos.
Knysna estuarine bay naturally supports the largest single area of the vulnerable species Zostera capensis in South Africa, which represents at least 25% and probably nearer 40% of the country’s total (Adams 2016). This seagrass covers nearly 40% of the whole Knysna system (Wasserman et al. 2020) and extends over a salinity gradient extending down to < 5 (Maree 2000). In this respect, distribution of Z. capensis at Knysna contrasts markedly with those of related Zostera spp (marina and noltei) which, at least in non-tidal lagoonal situations, have been described as being critically affected by salinity (positively) and nearness to sources of freshwater (negatively) (Boscutti et al. 2015). The height on the shore to which Z. capensis extends at Knysna is also relatively large: it routinely occurs right up to the interface with salt-marsh [0.2 m below HWN (Maree 2000)] and in some areas well into the fringing marsh vegetation (pers. obs.). Narrow, up-to-3 m-deep, marina canals flanked by tall buildings would not appear to be the ideal habitat for a permanently-submerged, light-loving seagrass (Lee et al. 2007), and together with its vertical and horizontal distribution this suggests that the Knysna population may be particularly tolerant of environmental extremes.
Knysna also supports the highest macrobenthic biodiversity of any known South African estuary (Turpie 2004; Turpie et al. 2004), containing some 40+% of that country’s estuarine total (Claassens et al. 2020); and within the Knysna system the most biodiverse region is its marine embayment wherein is located the Thesen marina (Barnes 2021). Clearly the construction of even marina estates on brownfield land within ‘nationally important’ areas of the ‘highest conservation priority’, as is the case with Knysna (Turpie et al. 2002; van Niekerk et al. 2019), is to say the least questionable (Barnes 1991; Airoldi and Beck 2007), not only because of the disturbance associated with the construction processes themselves (Iannuzzi et al. 1996; Prosser et al. 2018) and the danger of contamination (Leger et al. 2016), but also because of the effects thereafter of boat traffic (Sagerman et al. 2020; Carreño and Lloret 2021). In such a context, however, it is more than a little ironic that the region of highest Zostera-associated macrobenthic biodiversity within Knysna’s marine embayment, and hence in the whole of South Africa, is located in an artificial gabion-lined canal system, and that the canals also shelter two IUCN-listed endangered species. Although the seahorse Hippocampus capensis is often associated with Zostera capensis, the region of the Thesen canal system supporting the enhanced Zostera-associated invertebrate biodiversity is not the same one as those supporting enhanced densities of the endangered seahorse (Claassens 2016): the zone of increased invertebrate biodiversity near the mouth of the canal system is an additional conservation benefit to that of the seahorse population.
The Thesen marina system, however, is not likely to be typical of coastal marinas. Data are not readily available but personal experience suggests that almost all coastal marina estates have been constructed on greenfield marine sites (i.e. in previously natural habitat), the construction of which obliterates natural intertidal and subtidal habitat (Airoldi and Beck 2007). On the contrary, the Thesen marina was developed across an entire polluted brownfield terrestrial site, an original sand-bank island (Paarden Island) that had been purchased in 1904 to locate a sawmill and timber processing plant which it supported for almost a century, the island being renamed Thesen Island after the timber and ship-owning family concerned (Mulder 2008). The through-flow artificial canal system created on its decontamination from toxic substances used in wood processing (As, Cr, Cu, creosote tars, etc.) and redevelopment into a series of 19 small interlinked islands (Knynsa Museums 2022) was therefore a net gain to the estuarine bay’s subtidal aquatic habitat.
This may be unusual in the context of marina estates, but man-made coastal ponds and channels elsewhere have proved valuable habitats for the conservation of lagoonal and other coastal invertebrates (Barnes 1991; Allen et al. 1995), fish (Cavraro et al. 2021), and shorebirds (Rehfisch 1994). Most of the British coastal lagoons of conservation interest, for example, have been formed in one way or another by man, including excavated pits and scrapes (Barnes 1989; and see Herbert et al. 2018). Equivalent similar aquatic pools or channels that form small islands of high biodiversity in unlikely urbanised settings, including those of the seagrassed areas in the Thesen marina canals, could be categorised as novel ecosystems in the sense of Hobbs et al. (2009) produced by land-use change, and could potentially be the subject of ‘other effective area-based conservation measures’ (OECMs) (Pinto et al. 2021) creating, for example, soft-sediment equivalents of the ‘artificial marine micro-reserves’ of García-Gómez et al. (2015), Ostalé-Valriberas et al. (2022), etc.
To the best of our knowledge, this is the first time that the abundance and biodiversity of seagrass macrobenthos within any marina-estate system, greenfield or brownfield, has been surveyed, and hence there is no body of equivalent information from elsewhere with which it can be compared and no means of judging whether the brownfield Knysna situation is likely to be representative. Meanwhile, the appetite for coastal marina development is unlikely to decrease and the economic benefits of such schemes will often continue to be perceived as outweighing the disadvantages to coastal ecosystems, even to those of major international conservation significance. Three general features related to the present study, however, seem relevant to the future conservation of seagrass-associated biodiversity in such human-influenced areas. (i) A casual survey of developed areas of coastline suggests that the former Thesen Island at Knysna is far from being alone as a brownfield or derelict site on the terrestrial side of the land/sea interface (e.g. Leger et al. 2016; Fernandez et al. 2020); (ii) The potential advantages of small artificial coastal water bodies for conservation of shallow-water marine, lagoonal and brackish-water species seem well established and many have been incorporated into reserves of some form (Bamber 2010); and (iii) Design criteria for such artificial coastal habitats to serve as valuable areas of conservation are readily available (Bamber et al. 1993; Dafforn et al. 2015a, b) and could easily be included in the design of brownfield-estate waterways and, most importantly, in subsequent management protocols (Wauchope et al. 2022). Much is made locally of the beneficial effects of the Thesen marina canals on populations of the rare Knysna seahorse, suggesting that measures that lead to conservation of local biodiversity have good publicity value and public-relations advantages within the context of the operation of a commercial concern that has added to coastal urbanisation. Taken together these surely emphasise that, if planned, decontaminated and located appropriately (e.g. Epsilon 2001), brownfield-site marina development and conservation of coastal marine biodiversity need not be antithetical; and restored degraded areas can be of positive benefit to the local aquatic environment (e.g. Lemasson et al. 2020). In addition, any concomitantly lessened need for greenfield intertidal space would also be a reprieve for threatened shore-bird populations. The sustainable use of impacted coastlines is of major global concern (Bardos et al. 2020), and the comment of Adorjan et al. (2019) with respect to riverside development that ‘brown is the new green’ may apply equally well to the coast.
Data Availability
all new and historical data generated or analysed during this study are included in this published article and its supplementary information file.
References
Adams JB (2016) Distribution and status of Zostera capensis in South African estuaries - A review. S Afr J Bot 107:63–73. doi:https://doi.org/10.1016/j.sajb.2016.07.007
Adorjan A, Pecze A, Szilágyi K (2019) ‘Brown’ is the new ‘green’: Post-industrial sites as potential in the development of the green infrastructure on the riverfront of Budapest, Hungary. Proc Fábos Conf on Landscape and Greenway Planning 6:9. doi:https://doi.org/10.7275/pfeh-sm61
Airoldi L, Balata D, Beck MW (2008) The Gray zone: Relationships between habitat loss and marine diversity and their applications in conservation. J Exp Mar Biol Ecol 366:8–15. doi:https://doi.org/10.1016/j.jembe.2008.07.034
Airoldi L, Beck MW (2007) Loss, status and trends for coastal marine habitats of Europe. Oceanogr Mar Biol Ann Rev 45:345–405. doi:https://doi.org/10.1201/9781420050943.ch7
Albano PG, Sabelli B, Bouchet P (2011) The challenge of small and rare species in marine biodiversity surveys: microgastropod diversity in a complex tropical coastal environment. Biodivers Conserv 20:3223–3237. doi: https://doi.org/10.1007/s10531-011-0117-x
Allen JR, Wilkinson SB, Hawkins SJ (1995) Redeveloped docks as artificial lagoons: The development of brackish-water communities and potential for conservation of lagoonal species. Aquat Conserv 5:299–309. doi:https://doi.org/10.1002/aqc.3270050405
Alves-Pinto HA, Geldmann J, Jonas H, Maioli V, Balmford A, Latawiec AE, Crouzeilles R, Strassburg B (2021) Opportunities and challenges of other effective area-based conservation measures (OECMs) for biodiversity conservation. Perspect Ecol Conserv 19:115–120. doi:https://doi.org/10.1016/j.pecon.2021.01.004
Antão LH, Magurran AE, Dornelas M (2021) The shape of species abundance distributions across spatial scales. Front Ecol Evol 9:626730. doi:https://doi.org/10.3389/fevo.2021.626730
Baird D, Marais JFK, Wooldridge T (1981) The influence of a marina canal system on the ecology of the Kromme estuary, St Francis Bay. S Afr J Zool 16:21–34. doi:https://doi.org/10.1080/02541858.1981.11447729
Bamber RN (2010) Coastal Saline Lagoons and the Water Framework Directive. Natural England Commissioned Reports No. 039
Bamber RN, Batten SD, Bridgwater ND (1993) Design criteria for the creation of brackish lagoons. Biodivers Conserv 2:127–137. doi:https://doi.org/10.1007/BF00056129
Bardos P, Spencer KL, Ward RD, Maco BH, Cundy AB (2020) Integrated and sustainable management of post-industrial coasts. Front Environ Sci 8:86. doi:https://doi.org/10.3389/fenvs.2020.00086
Barnes RSK (1989) The coastal lagoons of Britain: An overview and conservation appraisal. Biol Conserv 49:295–313. doi:https://doi.org/10.1016/0006-3207(89)90049-9
Barnes RSK (1991) European estuaries and lagoons: a personal overview of problems and possibilities for conservation and management. Aquat Conserv 1:79–87. doi:https://doi.org/10.1002/aqc.3270010107
Barnes RSK (2014) The nature and location of spatial change in species assemblages: a new approach illustrated by the seagrass macrofauna of the Knysna estuarine bay, South Africa. Trans R Soc S Afr 69:75–80. doi:https://doi.org/10.1080/0035919X.2014.899277
Barnes RSK (2018) Little-known and phylogenetically obscure South African estuarine microgastropods (Mollusca: Truncatelloidea) as living animals. J Nat Hist 52:87–113. doi:https://doi.org/10.1080/00222933.2017.1408867
Barnes RSK (2019) Local patchiness of macrobenthic faunal abundance displays homogeneity across the disparate seagrass systems of an estuarine bay. Mar Environ Res 148:99–107. doi: https://doi.org/10.1016/j.marenvres.2019.05.001
Barnes RSK (2021) Patterns of seagrass macrobenthic biodiversity in the warm-temperate Knysna estuarine bay, Western Cape: A review. Aquat Ecol 55:327–345. doi:https://doi.org/10.1007/s10452-021-09848-3
Barnes RSK, Claassens L (2020) Do beds of subtidal estuarine seagrass constitute a refuge for macrobenthic biodiversity threatened intertidally? Biodivers Conserv 29:3227–3244. doi:https://doi.org/10.1007/s10531-020-02019-0
Beck J, Holloway JD, Schwanghart W (2013) Undersampling and the measurement of beta diversity. Methods Ecol Evol 4:370–382. doi:https://doi.org/10.1111/2041-210x.12023
Bishop MJ, Mayer-Pinto M, Airoldi L, Firth LB, Dafforn KA (2017) Effects of ocean sprawl on ecological connectivity: impacts and solutions. J Exp Mar Biol Ecol 492:7–30. doi:https://doi.org/10.1016/j.jembe.2017.01.021
Blake RE, Duffy JE, Richardson JP (2014) Patterns of seagrass community response to local shoreline development. Estuar Coast 37:1549–1651. doi:https://doi.org/10.1007/s12237-014-9784-7
Blowes SA, Daskalova GN, Dornelas M, Chase JM (2022) Local biodiversity change reflects interactions among changing abundance, evenness and richness. bioRxiv. doi:https://doi.org/10.1101/2021.08.29.458087
Boscutti F, Marcorin I, Sigura M, Bressan E, Tamberlich F, Vianello A, Casolo V (2015) Distribution modeling of seagrasses in brackish waters of Grado-Marano lagoon (Northern Adriatic Sea). Estuar Coast Shelf Sci 164:183–193. doi:https://doi.org/10.1016/j.ecss.2015.07.035
Bosch NE, Gonçalves JMS, Tuya F, Erzini K (2017) Marinas as habitats for nearshore fish assemblages: comparative analysis of underwater visual census, baited cameras and fish traps. Sci Mar 81:159–169. doi:https://doi.org/10.3989/scimar.04540.20A
Bouchet P, Lozouet P, Maestrati P, Heros V (2002) Assessing the magnitude of species richness in tropical marine environments: exceptionally high numbers of molluscs at a New Caledonia site. Biol J Linn Soc 75:421–436. doi: https://doi.org/10.1046/j.1095-8312.2002.00052.x
Bouchoucha M, Darnaude AM, Gudefin A, Neveu R, Verdoit-Jarraya M, Boissery P, Lenfant P (2016) Potential use of marinas as nursery grounds by rocky fishes: insights from four Diplodus species in the Mediterranean. Mar Ecol Progr Ser 547:193–209. doi:https://doi.org/10.3354/meps11641
Bulleri F, Chapman MG (2010) The introduction of coastal infrastructure as a driver of change in marine environments. J Appl Ecol 47:26–35. doi:https://doi.org/10.1111/j.1365-2664.2009.01751.x
Carreño A, Lloret J (2021) Environmental impacts of increasing leisure boating activity in Mediterranean coastal waters. Ocean Coast Manage 209:105693. doi:https://doi.org/10.1016/j.ocecoaman.2021.105693
Cavraro F, Finotti G, Rossato G, Zucchetta M, Facca C, Malavasi S (2021) A comparative analysis of habitat quality between artificial and natural creeks in the Mediterranean killifish Aphanius fasciatus: Implications for conservation. Aquat Conserv 31:1311–1321. doi:https://doi.org/10.1002/aqc.3532
Chou LM, Toh KB, Ng CSL (2015) Coastal urbanization impacts on biodiversity - the case of marinas in Singapore. The Asian Conference on Sustainability, Energy and the Environment 2015 Official Conference Proceedings, 389–395. International Academic Forum, Aichi. ISSN:2186–2311
Claassens L (2016) An artificial water body provides habitat for an endangered estuarine seahorse species. Estuar Coast Shelf Sci 180:1–10. doi:https://doi.org/10.1016/j.ecss.2016.06.011
Claassens L, Barnes RSK, Wasserman J, Lamberth SJ, Miranda NAF, van Niekerk L, Adams JB (2020) Knysna Estuary health: ecological status, threats and options for the future. Afr J Aquat Sci 45:65–82. doi:https://doi.org/10.2989/16085914.2019.1672518
Claassens L, Hodgson AN (2018) Monthly population density and structure patterns of an endangered seahorse Hippocampus capensis: a comparison between natural and artificial habitats. J Fish Biol 92:2000–2015. doi:https://doi.org/10.1111/jfb.13639
Dafforn KA, Glasby TM, Airoldi L, Rivero NK, Mayer-Pinto M, Johnston EL (2015a) Marine urbanization: an ecological framework for designing multifunctional artificial structures. Front Ecol Environ 13:82–90. doi:https://doi.org/10.1890/140050
Dafforn KA, Mayer-Pinto M, Morris RL, Waltham NJ (2015b) Application of management tools to integrate ecological principles with the design of marine infrastructure. J Environ Manage 158:61–73. doi:https://doi.org/10.1016/j.envman.2015.05.001
Davidson IC, Crook AC, Barnes DKA (2004) Quantifying spatial patterns of intertidal biodiversity: is movement important? Mar Ecol 25:15–34. doi:https://doi.org/10.1111/j.1439-0485.2004.00015.x
Day JH, Millard NAH, Harrison AD (1951) The ecology of South African estuaries. Part III. Knysna: A clear open estuary. Trans R Soc S Afr 33:367–413. doi:https://doi.org/10.1080/00359195109519891
Day JH (ed) (1981) Estuarine Ecology with particular reference to southern Africa. Balkema, Rotterdam
Dethier MN, Schoch GC (2006) Taxonomic sufficiency in distinguishing natural spatial patterns on an estuarine shoreline. Mar Ecol Progr Ser 306:41–49. doi:https://doi.org/10.1111/J.1439-0485.2004.00015.X
de Villiers CJ, Hodgson AN, Forbes AT (1999) Studies on estuarine macroinvertebrates. In: Allanson BR, Baird D (eds) Estuaries of South Africa. Cambridge University Press, Cambridge, pp 167–207
de Villiers NM, Harasti D, Hodgson AN, Claassens L (2021) A comparison of the fauna in eelgrass and erosion control structures in a warm temperate Southern African estuary. Reg Stud Mar Sci 44:101757. doi:https://doi.org/10.1016/j.rsms.2021.101757
Duarte CM, Marbà N, Santos R (2004) What may cause loss of seagrasses? In: Borum J, Duarte CM, Krause-Jensen D, Greve TM (eds) European Seagrasses: An Introduction to Monitoring and Management. EU Monitoring & Managing of European Seagrass Project Report EVK3-CT-2000-00044. ISBN 87-89143-21-3, pp 24–32
Duggan JE, Airoldi L, Chapman MG, Walker SJ, Schlacher T (2011) Estuarine and coastal structures: Environmental effects, a focus on shore and nearshore structures. In: Kennish MJ, Elliott M (eds), Treatise on Estuarine and Coastal Science 8 Human-induced Problems (Uses and Abuses), Academic Press, pp 17–41. doi:https://doi.org/10.1016/B978-0-12-374711-2.8002-0
Duvenage IR, Morant PD (1984) Estuaries of the Cape, Part II: Synopses of Available Information on Individual Systems No. 31: Keurbooms/Bitou System and Piesang. CSIR Research Report 430. NRIO/CSIR, Stellenbosch
Epsilon (2001) Massachusetts Clean Marina Guide. Strategies to Reduce Environmental Impacts. Massachusetts Office of Coastal Zone Management, Boston Ma
Fernandez A, Figueira de Sousa J, Coasta JP, Neves B (2020) Mapping stakeholder perception on the challenges of brownfield sites’ redevelopment in waterfronts: the Tagus Estuary. Eur Plan Stud 28:2447–2464. doi:https://doi.org/10.1080/09654313.2020.1722985
Foster V, Giesler RJ, Wilson AMW, Nall CR, Cook EJ (2016) Identifying the physical features of marina infrastructure associated with the presence of non-native species in the UK. Mar Biol 163:173. doi:https://doi.org/10.1007/s00227-016-2941-8
García-Gómez JC, Guerra-García JM, Espinosa F, Maestre MJ, Rivera-Ingraham G, Fa D, González AR, Ruiz-Tabares AR, López-Fé CM (2015) Artificial Marine Micro-Reserves Networks (AMMRNs): an innovative approach to conserve marine littoral biodiversity and protect endangered species. Mar Ecol 36:259–277. doi:https://doi.org/10.1111/maec.12167
Gerwing TG, Cox K, Allen Gerwing AM, Campbell L, Macdonald T, Dudas SE, Juanes F (2020) Varying intertidal invertebrate taxonomic resolution does not influence ecological findings. Estuar Coast Shelf Sci 232:106516. doi:https://doi.org/10.1016/j.ecss.2019.106516
Gotelli NJ, Colwell RK (2001) Quantifying biodiversity: procedures and pitfalls in the measurement and comparison of species richness. Ecol Lett 4:379–391. doi:https://doi.org/10.1046/j.1461-0248.2001.00230.x
Griffiths CL, Robinson TB, Mead A (2009) The status and distribution of marine alien species in South Africa. In: Rilov G, Crooks JA (eds) Biological Invasions in Marine Ecosystems. Springer, Berlin, pp 393–408
Griffiths CL, Robinson TB, Mead A (2011) The alien and cryptogenic marine crustaceans of South Africa. In: Gatil BS, Clark PF, Carlton JT (eds) In the Wrong Place - Alien Marine Crustaceans: Distribution, Biology and Impacts. Springer, Dordrecht, pp 269–282. doi:https://doi.org/10.1007/978-94-007-0591-3_8.
Guerra-García JM, García-Gómez JC (2005) Oxygen levels versus chemical pollutants: do they have similar influence on macrofaunal assemblages? A case study in a habbour with opposing entrances. Environ Poll 135:281–291. doi:https://doi.org/10.1016/j.envpol.2004.10.004
Guerra-García JM, Navarro-Barranco C, Ros M, Moreira J (2021) Ecological quality assessment of marinas: An integrative approach combining biological and environmetal data. J Environ Manag 286:112237. doi:https://doi.org/10.1016/j/jenvman.2021.112237
Hammer Ø, Harper DAT, Ryan PD (2019) PAST: Paleontological statistics software package for education and data analysis
Herbert RJH, Broderick LG, Ross K, Moody C, Cruz T, Clarke L, Stillman RA (2018) Artificial coastal lagoons at solar salt-working sites: A network of habitat for specialised, protected and alien biodiversity. Estuar Coast Shelf Sci 203:1–16. doi:https://doi.org/10.1016/j.ecss.2018.01.015
Hobbs RJ, Higgs E, Harris JA (2009) Novel ecosystems: implications for conservation and restoration. Trend Ecol Evol 24:599–605. doi:https://doi.org/10.1016/j.tree.2009.05.012
Iannuzzi TJ, Weinstein MP, Sellner KG, Barrett JC (1996) Habitat disurbance and marina development: An assessment of ecological effects. I. Changes in primary production due to dredging and marina construction. Estuar Coast 19:257–271. doi;10.2307/1352231
Knysna Museums (2022) Thesen Islands Heritage Walk brochure. Available at https://www.knysnamuseums.co.za//media/doc/hl/thesen_heritage_walk_panels.pdf
Lee K-S, Park SR, Kim YK (2007) Effects of irradiance, temperature and nutrients on growth dynamics of seagrasses: A review. J Exp Mar Biol Ecol 350:144–175. doi:https://doi.org/10.1016/j.jembe.2007.06.016
Legendre P, Legendre L (1998) Numerical Ecology, 2 edn. English Edn. Elsevier, Amsterdam
Leger C, Balch C, Essex S (2016) Understanding the planning challenges of brownfield development in coastal urban areas of England. Plan Pract Res 31:119–131. doi:https://doi.org/10.1080/02697459.2016.1146428
Lemasson AJ, Pettit LR, Smith RK, Sutherland WJ (2020) Subtidal benthic invertebrate conservation. In: Sutherland WJ, Dicks LV, Petrovan SO, Smith RK (eds) What Works in Conservation 2020. Open Book Publishers, Cambridge, pp 635–732
Leopardas V, Uy W, Nakaoka M (2014) Benthic macrofaunal assemblages in multispecific seagrass meadows of the southern Philippines: Variation among vegetation dominated by different seagrass species. J Exp Mar Biol Ecol 457:71–80. doi:https://doi.org/10.1016/j.jembe.2014.04.006
Lloyd M (1967) Mean crowding. J Anim Ecol 36:1–30. doi:https://doi.org/10.2307/3012
Macdonald TA, Burd BJ, Macdonald VI, van Roodselaar A (2010) Taxonomic and feeding guild classification for the marine benthic macroinvertebrates of the Strait of Georgia, British Columbia. Can Techn Rep Fish Aquat Sci No 2874:63
Maree B (2000) Structure and status of the intertidal wetlands of the Knysna Estuary. Trans R Soc S Afr 55:163–176. doi:https://doi.org/10.1080/00359190009520441
Maxted JR, Weisberg SB, Chaillou JC, Eskin RA, Kutz EW (1997) The ecological condition of dead-end canals of the Delaware and Maryland Coastal Bays. Estuaries 20:319–327. doi:https://doi.org/10.2307/1352347
Mineur F, Cook EJ, Minchin D, Bohn K, Macleod A, Maggs CA (2012) Changing coasts: marine aliens and artificial structures. Oceanogr Mar Biol Ann Rev 50:189–234. doi:https://doi.org/10.1201/b12157-5
Momota K, Hosokawa S (2021) Potential impacts of marine urbanization on benthic macrofaunal diversity. Sci Rep 11:4028. doi:https://doi.org/10.1038/s41598-021-83597-z
Mulder C(2008) Foreword. In: Thesen Islands, pp 10–25. Quivertree, Cape Town. Reprinted in Ndubisi FO (ed) (2014) The Ecological Design and Planning Reader, Island Press, Washington DC, pp 456–460. doi:https://doi.org/10.5822/978-1-61091-491-8_39
Murray NJ, Phinn SR, DeWitt M, Ferrari R, Johnston R, Lyons MB, Clinton N, Thau D, Fuller RA (2019) The global distribution and trajectory of tidal flats. Nature 565:222–225. doi:https://doi.org/10.1038/s41586-018-0805-8
Murray NJ, Worthington TA, Bunting P, Lyons N (2022) High-resolution mapping of losses and gains of Earth’s tidal wetlands. Science 376:744–749. doi:https://doi.org/10.1126/science.abm9583
Natchez DS(2018) On the waterfront: all mixed up — marinas within mixed-use development. https://www.marinadockage.com/waterfront-mixed-marinas-within-mixed-use-development/
Ostalé-Valriberas E, Sempere-Valverde J, Pavón-Paneque A, Coppa S, Espinosa F, García-Gómez JC (2022) Artificial marine micro-reserves as a new ecosystem-based management tool for marine conservation: The case of Patella ferruginea (Gastropoda, Patellidae), one of the most endangered marine invertebrates of the Mediterranean. Mar Policy 136:104917. doi:https://doi.org/10.1016/j.marpol.2021.104917
Passy SI (2016) Abundance inequality in freshwater communities has an ecological origin. Amer Nat 187:505–516. doi:https://doi.org/10.1086/685424
Perissinotto R, Miranda NAF, Raw JL, Peer N (2014) Biodiversity census of Lake St Lucia, iSimangaliso Wetland Park (South Africa): Gastropod molluscs. ZooKeys 440:1–43. doi:https://doi.org/10.3897/zookeys.440.7803
Prosser DJ, Nagel JL, Jordan TE, Seitz RD, Weller DE, Whigham DF (eds) (2018) Impacts of coastal land use and shoreline armoring on estuarine ecosystems. Estuar Coast 41:Suppl 1
Rehfisch MM (1994) Man-made lagoons and how their attractiveness to waders might be increased by manipulating the biomass of an insect benthos. J Appl Ecol 31:383–401. doi:https://doi.org/10.2307/2404552
Rivero NK, Dafforn KA, Coleman MA, Johnston EL (2013) Environmental and ecological changes associated with a marina. Biofouling 29:803–815. doi:https://doi.org/10.1080/08927014.2013.805751
Sagerman J, Hansen JP, Wilström SA (2020) Effects of boat traffic and mooring infrastructure on aquatic vegetation: A systematic review and meta-analysis. Ambio 49:517–530. doi:https://doi.org/10.1007/s13280-019-01215-9
Sheppard C (ed) (2019) World Seas: An Environmental Evaluation, 2 Edn. Academic Press
Turpie JK(2004) An updated importance rating of all South African estuaries. In: Turpie J (ed) Contributions to information requirements for the implementation of resource directed measures for estuaries. 1. Improving the biodiversity importance rating of South African estuaries. Report to Water Research Commission on behalf of the Consortium for Estuarine Research and Management. Water Research Commission Report 1247/1/04, pp 109–120
Turpie JK, Adams JB, Joubert A, van Niekerk L (2002) Assessment of the conservation priority status of South African estuaries for use in management and water allocation. Water SA 28:191–206 ISSN 0378–4738
Turpie JK, Clark B, Savy C(2004) Predicting invertebrate species richness on the basis of broadscale estuary characteristics. In: Turpie J (ed) Contributions to information requirements for the implementation of resource directed measures for estuaries. 1. Improving the biodiversity importance rating of South African estuaries. Report to Water Research Commission on behalf of the Consortium for Estuarine Research and Management. Water Research Commission Report 1247/1/04, pp 80–108
Valdor PF, Gómez AG, Juanes JA, Méndez G (2019) A global atlas of the environmental risk of marinas on water quality. Mar Poll Bull 149:110661. doi:https://doi.org/10.1016/j.marpolbul.2019.110661
van Niekerk L, Adams JB, Lamberth SJ, MacKay CF, Taljaard S, Turpie JK, Weerts SP, Raimondo DC (eds) (2019) South African National Biodiversity Assessment 2018: Technical Report. 3. Estuarine Realm. CSIR/SANBI Report CSIR/SLPA/EM/EXP/2019/0062/A; SANBI/NAT/NBA2018/2019/Vol3/A
Waltham NJ, Connolly RM (2011) Global extent and distribution of artificial, residential waterways in estuaries. Estuar Coast Shelf Sci 94:192–197. doi:https://doi.org/10.1016/j.ecss.2011.06.003
Warwick RM, Dashfield SL, Somerfield PJ (2006) The integral structure of a benthic infaunal assemblage. J Exp Mar Biol Ecol 330:12–18. doi:https://doi.org/10.1016/j.jembe.2005.12.013
Wasserman J, Claassens L, Adams JB (2020) Mapping subtidal estuarine habitats with a remotely operated underwater vehicle (ROV). Afr J Mar Sci 42:123–128. doi:https://doi.org/10.2989/1814232X.2020.1731598
Wauchope HS, Jones JPG, Geldman J, Sutherland WJ (2022) Protected areas have a mixed impact on waterbirds, but management helps. Nature 605:103–107. doi:https://doi.org/10.1038/s41586-022-04617-0
Whitfield AK (1989) The benthic invertebrate community of a southern Cape estuary: structure and possible food sources. Trans R Soc S Afr 47:159–179. doi:https://doi.org/10.1080/00359198909520160
Acknowledgements
We are most grateful to: Rhodes University Research Committee for financial support; Thesen Islands Home Owners Association and Thesen Harbour Town for permission to work within the marina estate; the Rondevlei Office of SANParks Scientific Services and the Knysna Area Manager, Megan Taplin, for permission to undertake fieldwork in the Knysna Section of the Garden Route National Park under Permit BARN-RSK/2019-034; and both SANParks Knysna Lakes Office and the Knysna Basin Project for laboratory facilities. We are also grateful for the editorial and reviewers’ comments that led to improvement of the article.
Funding
No external or specific funding was received for conducting this study.
Author information
Authors and Affiliations
Contributions
Authorship contributions: RSKB conceived and designed this study; data collection was performed by RSKB, LC and JS, and data analysis by RSKB; the first draft of the manuscript was written by RSKB and all authors commented on later versions; all authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Ethical approval
All applicable international, national and/or institutional guidelines for sampling, care and experimental use of organisms for the study were followed and all necessary permissions and approvals were obtained in respect of the original collections of the data.
Consent for publication
all authors consent to publication.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Barnes, R.S., Claassens, L. & Seath, J. Where ecologically ‘tis better to go brown than green: enhanced seagrass macrobenthic biodiversity within the canals of a brownfield coastal marina. Biodivers Conserv 31, 2981–2997 (2022). https://doi.org/10.1007/s10531-022-02468-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10531-022-02468-9