Abstract
Hematopoietic system transports all necessary nutrients to the whole organism and provides the immunological protection. Blood cells have high turnover, therefore, this system must be dynamically controlled and must have broad regeneration potential. In this review, we summarize how this complex system is regulated by the heme oxygenase-1 (HO-1)—an enzyme, which degrades heme to biliverdin, ferrous ion and carbon monoxide. First, we discuss how HO-1 influences hematopoietic stem cells (HSC) self-renewal, aging and differentiation. We also describe a critical role of HO-1 in endothelial cells and mesenchymal stromal cells that constitute the specialized bone marrow niche of HSC. We further discuss the molecular and cellular mechanisms by which HO-1 modulates innate and adaptive immune responses. Finally, we highlight how modulation of HO-1 activity regulates the mobilization of bone marrow hematopoietic cells to peripheral blood. We critically discuss the issue of metalloporphyrins, commonly used pharmacological modulators of HO-1 activity, and raise the issue of their important HO-1-independent activities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
HSC and their niche, hematopoiesis
Hematopoietic stem cells (HSC) produce all the blood cells throughout life in a process called hematopoiesis. The main characteristics of HSC are self-renewal and differentiation toward all cells among the hematopoietic blood lineages (reviewed in [1, 2]).
The rare HSC sit at the apex of the hierarchical structure of the hematopoietic system. In 1988 Weissman laboratory isolated murine HSC and showed that this cell population differentiates to all mature blood lineages in the irradiated mice [3]. Further research revealed three distinct subpopulations at the top of hematopoietic tree: long-term HSC (LT-HSC), short-term HSC (ST-HSC), and multipotent progenitors (MPP) [4]. While all of these populations have multipotent differentiation potential, only the LT-HSC can self-renew over the entire lifetime, what is progressively lost as they differentiate toward ST-HSC and MPP. MPP differentiate downstream into two types of oligopotent progenitors: common myeloid progenitors (CMP) and common lymphoid progenitors (CLP), where myeloid oligopotent progenitors will give rise to bipotent megakaryocyte-erythrocyte progenitors (MEP) and granulocyte–macrophage progenitors (GMP) [5, 6]. Those oligopotent progenitors will eventually differentiate to unipotent progenitors and ultimately will give rise to downstream diverse mature progenies [7]. Several studies based on single-cell transplantation assays questioned, however, the classic hematopoietic hierarchical differentiation tree and suggested that subpopulation of HSC may directly differentiate to lineage-biased progenitors, skipping the MPP stage [8, 9].
The proper function of HSC tightly depends on the bone marrow (BM) microenvironment named 'niche'. This specialized niche sustains the multipotency and self-renewal of HSC and prevents their exhaustion. Therefore, the niche is critical for maintaining the hematopoietic homeostasis and regulating the response of hematopoietic system in stress conditions [10].
The conceptualization of stem cell 'niche' as a specialized microenvironment within the BM referred to studies done a few decades earlier by Schofield (1978). He proposed that the niche regulates HSC properties of multipotency, self-renewal, and quiescence via specific signals [11]. Over the years this concept was consequently confirmed by numerous studies that demonstrated many cellular and non-cellular components of the HSC-niche. The niche complex milieu provides the HSC with the essential physical interaction and the molecular cues that are critical for the differentiation, maintenance, and localization of HSC.
HSC niche is composed of several cell types. First findings showed a crucial role of stromal cells, and identified clonal BM stromal cells which support the HSC self-renewal, and the maturation of both CMP and CLP [12]. Of different mesenchymal stromal cells (MSC), CXCL12-abundant reticular (CAR) cells are essential for maintaining the quiescence of HSC [13]. CAR phenotype highly overlaps with the later-described leptin receptor-expressing MSC (LepR +) [14]. While it was initially indicated that HSC niche locates near the endosteum [15, 16], further studies suggested that the majority of HSC reside in direct proximity of blood vessels rather than near bone surface [17,18,19].
The development of the transgenic mouse models and advanced microscopy techniques facilitated the identification of subsequent cellular elements of HSC niche as essential factors in regulating HSC function. These included hematopoietic cell types such as megakaryocytes [20], and non-hematopoietic cell types such as MSC, adipocytes and glial cells, as well as niche-derived growth factors and signaling molecules, along with their receptors [21, 22].
HSC progenies are also able to regulate HSC activities in a feedback loop. For instance, HSC quiescence can be regulated directly by megakaryocytes [20, 23,24,25]. Bruns et al. described HSC subpopulation associated with megakaryocytes, and reported that depletion of megakaryocytes may trigger HSC proliferation [23]. After exposure to a lethal dose of radiation, megakaryocytes drive the repair of HSC niche via osteolineage cell differentiation [24].
Despite the extensive studies on HSC niche, some questions are still unanswered and some mechanisms underlying the HSC-niche interaction within the BM and their function are incompletely understood.
The existence of clonal precursors for hematopoiesis was suggested already in 1960′s [26, 27] and since then HSC became one of the most intensively studied subjects of research. Nevertheless, there are still a lot of unanswered questions and controversies concerning their biology and differentiation and their interactions with their niche [28]. Among many factors that were shown to regulate this system was heme oxygenase-1 (HO-1).
Heme oxygenase-1
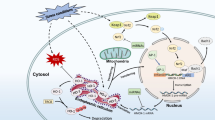
Heme oxygenase is an enzyme which degrades heme [29]. The products of the reaction catalyzed by heme oxygenase are equimolar amounts of carbon monoxide (CO), ferrous ions and biliverdin, which is subsequently converted to bilirubin by biliverdin reductase (BVR) [30]. Catalytic heme degradation requires an electron donor, that is NADPH provided by P450 cytochrome reductase and oxygen (reviewed in [31], (Fig. 1)).
There are two existing isoforms of heme oxygenase, HO-1 and HO-2 [32, 33], encoded by two different genes, HMOX1 and HMOX2 [31]. While HO-2 is a constitutive isoform, expressed mainly in the brain and testes [34, 35], HO-1 is induced by a variety of factors. The most obvious advantage of HO-1 activity is the removal of free heme, which is a known pro-oxidant, and a direct regulator of several transcription factors [36]. Moreover, heme can act as a damage-associated molecular pattern (DAMP) and activate innate immune response [37].
Of all the heme degradation products, CO seems to be the most important in the regulation of the immune system (reviewed in [31]). CO reduces the production of proinflammatory cytokines—IL (interleukin)-1, IL-6, TNFα (tumor necrosis factor α) and the expression of adhesion molecules, and simultaneously increases the production of anti-inflammatory IL-10 [38]. CO affects the transmission of signals from certain TLRs (Toll-like receptors), which are very important in the initiation of immune responses against pathogens [39]. CO can also act as an anti-apoptotic factor by modulating Fas/Fas ligand and Bcl-2 family [40].
The second of the heme degradation products, biliverdin, is almost instantly converted to bilirubin by BVR [41]. Bilirubin is a potent antioxidant and anti-inflammatory factor—it is an efficient scavenger of reactive oxygen species [42] and inhibits the adhesion molecules signaling [43, 44].
The last of the HO-1 reaction products, ferrous ions, can be considered harmful. However, the release of potentially pro-oxidant Fe2+ ions induces the expression of ferritin [45], which apart from sequestering iron, also can have an anti-apoptotic effects [46].
HO-1 might also directly regulate the cytokine expression [47]. Study by Ghoreschi et al. shows that fumarate treatment of DC upregulates HO-1, which is cleaved and translocated to the nucleus. In the nucleus HO-1 associates with AP-1 or NFκB binding sites in the IL-23p19 promoter, where it interferes with transcriptional activity. As a result, HO-1 decreases IL23p19 transcription [47].
A lot of data concerning the role of HO-1 in the normal and disease conditions were reported after the generation of genetically modified mice that lack the expression of HO-1 (HO-1−/−) [48]. HO-1−/− mice are affected by a chronic proinflammatory state and dysregulated iron homeostasis [48].
HO-1 deficiency was also reported in humans, for the first time by Yachie et al. in 1999 [49]. Since then there were only a few other cases described [50,51,52,53,54]. However, it is possible that due to the unspecific and variable symptoms, such as fever, hemolytic anemia, hematuria, proteinuria, hypertension and growth retardation, many other patients are undiagnosed. All of the five described patients from India had the same homozygotic R44X mutation [53], what suggests, that this mutation exists in the population. Unfortunately, the majority of the affected children died due to pathological changes in various organs [50, 53, 54]. It was shown that the absence of macrophages in the spleen and liver and in consequence the inability to remove senescent erythrocytes (RBC) and hemoglobin from the circulation is the main cause of the disease in HO-1 deficient mice [55]. Based on experiments in mice, Kovtunovych et. al suggested, that bone marrow transplantation could work as a possible treatment of HO-1 deficiency in humans [56]. Indeed, one patient was successfully treated with hematopoietic stem cell transplant from his sister [57].
HO-1 deficiency luckily seems to be extremely rare, but there are significant differences in HO-1 expression/activity in the human population due to the HMOX1 promoter polymorphism [58, 59]. The differences in the number of GT repeats affect HO-1 expression: shorter alleles result in the higher basal expression and stronger induction by the HO-1 inducers than the longer alleles [60] and were linked to the risk of various diseases, such as diabetes [61], multiple sclerosis [62], chemotherapy-induced neutropenia [63] and cardiovascular diseases [64, 65].
Role of HO-1 in HSC and progenitors
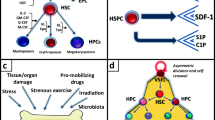
Due to the anti-inflammatory, antioxidative and anti-apoptotic properties of HO-1, it was hypothesized that HO-1 interferes with the HSC differentiation. The role of HO-1 in steady-state regulation of HSC is supported by our study based on HO-1 deficient mice (HO-1−/−) [66] (Fig. 2). The HO-1−/− mice have increased numbers of strictly phenotypically defined LT-HSC, as well as MPP. Moreover, the LT-HSC from HO-1−/− mice have a higher proliferation rate and show features of premature aging on global transcriptional and functional level [66]. This is connected with the increased DNA damage [66]. Although the altered biology of HSC in HO-1−/− mice is evident, we suggest that it results at least in part, from the role of HO-1 in the BM niche (discussed in the next chapter).
Another study employed double mutant HO-1−/−Bach1−/− mice [67]. In this model, the frequency of hematopoietic stem and progenitor cells (HSPC) is also increased, but the number of the common myeloid progenitors is decreased. This indicates that HO-1/Bach1 pathway is necessary for the commitment of multipotent progenitors to myeloid lineage [67]. However, the study did not analyze whether the same effect is observed in HO-1−/−Bach1+/+ model. Therefore, the direct role of HO-1 on the differentiation blockage at the CMP stage still has to be clarified, especially because HO-1−/−Bach1+/+ mice have higher numbers of circulating monocytes and granulocytes [66], what may seem inconsistent with reduced numbers of CMP in HO-1−/−Bach-1−/− mice.
Bach1 and Bach2 are DNA-binding proteins, which work mainly as transcriptional repressors [68]. Heme regulates Bach1 expression by binding to Bach1 protein and inducing its nuclear export [69]. Furthermore, heme was shown to induce the ubiquitination and proteasomal degradation of Bach1 [70]. Bach2 is repressing myeloid genes by direct binding to their promoters, thus promoting B cell development [71]. On the other hand, Bach1 and Bach2 redundantly repress Hmox1 expression [72]. Heme binds to Bach1 and Bach2 and inhibits their activity, providing the negative feedback loop for the control of Hmox1 expression.
HO-1 is important for the proper function of HSC not only in steady-state conditions but also under stress. HO-1 expression in HSPC in steady-state levels is low, but increases in hematopoietic stress, such as myeloablation. Paradoxically, a study done on HO-1 heterozygous mice (HO-1−/+) showed that these mice recovered after the 5-FU-induced myeloablation faster than HO-1+/+ mice, which was linked to increased proliferation of HSPC [73]. However, despite the faster response to myeloablation, HSC in HO-1−/+ mice lost the potential to reconstitute irradiated recipients after serial transplantation [73]. Thus, it suggests that HO-1 regulates how HSPC respond to stress stimuli and protects them from exaggerated proliferation and premature exhaustion.
Heme is an important regulator of erythropoiesis, therefore HO-1, as a heme degrading enzyme, was also suggested to modulate erythropoiesis [74]. The early evidence pointing to the potential role of HO-1 in erythropoiesis was presented by Abraham et al. [75, 76]. While the initial report based on K562 cell line suggests that HO-1 level in erythroid cells might be negligible [77], further studies done on mouse model evidenced that HO-1 is expressed in erythroblasts and that its expression is increasing with erythroid differentiation [78]. It was also shown that both downregulation and upregulation of HO-1 dysregulates proper erythroid differentiation [78].
Importantly, HO-1−/− mice present several disturbances in steady-state hematopoiesis and have microcytic anemia [79]. These erythroid defects may be caused not only by the lack of HO-1 in erythroid lineage but also by the lack of HO-1 in macrophages. The formation of erythroid cells occurs in so-called erythroblastic islands, that are composed by centrally located macrophage and adherent, differentiating erythroblasts [80]. The macrophages within the erythroblastic islands highly express HO-1, and HO-1-deficiency reduces their number and disturbs the formation of erythroblastic islands [79]. The crucial role of macrophages in erythroblastic islands may be connected with the enzymatic product of HO-1 reaction—carbon monoxide (CO) [79]. The in vitro model indicated that in low-oxygen conditions, that are typical for BM, CO prevented the death of erythroid precursors and triggered erythroid differentiation [81].
Finally, HO-1 plays an important role in stress hematopoiesis. In the transplantation model, which is used to simulate stress conditions, irradiated recipient mice that received HO-1+/− cells had lower numbers of erythroblasts. This was linked with an increased level of TNFα in the splenic macrophages and decreased CD49d expression levels in proerythroblasts [82].
Upstream from HO-1, erythropoiesis is regulated by Bach1 and Bach2 [83, 84]. Bach1−/−Bach2−/− mice have erythroblast-maturation disorder, which is connected with HO-1 de-repression. Moreover, downregulation of Bach1 and Bach2 during infection inhibits erythropoiesis, allowing for enhanced expression of myeloid genes and thus shifting the hematopoiesis towards the production of innate immunity cells [83, 84].
Role of HO-1 in HSC niche
Endothelial cells
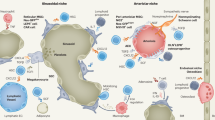
HSC and endothelial cells (EC) are closely related from the very beginning of fetal development. Endothelial cells are one of the most crucial regulators of HSC niche in the bone marrow. At the same time, endothelial cells are one of the cell types, in which HO-1 plays a critical role. Since there are comprehensive reviews that cover the topic of the role of HO-1 in endothelial cells [64, 85,86,87], we only highlight the most important issues.
HO-1 protects endothelial cells from apoptosis [88], and this is dependent on the generation of CO [89]. In general, HO-1 can act as a pro- and anti-proliferative factor, depending on the cell type, tissue and health/disease status. However, in the case of endothelial cells, HO-1 increases their proliferation [60, 90]. Because of its influence on EC proliferation, but also due to the modulation of expression and activity of proangiogenic factors, such as SDF-1 and VEGF, HO-1 is an important regulator of angiogenesis (reviewed in [91]). Furthermore, HO-1 can inhibit inflammation by decreasing the expression of adhesion molecules (E-selectin and VCAM-1) on the surface of endothelial cells [92]. This occurs through the inhibition of NF-κB [93].
The importance of HO-1 for the proper function of endothelial cells is further evidenced by the human cases of HO-1 deficiency—the affected children suffered from severe endothelial cell injury due to oxidative stress [49].
Mesenchymal stromal cells
Mesenchymal stromal cells (MSC) are also known by different names, such as multipotent stromal cells or—imprecisely—mesenchymal stem cells [94]. Although they can be found in different tissues, such as umbilical cord, adipose tissues, and placenta, the major source of MSC used in research is the bone marrow [95]. MSC can differentiate to osteoblasts, chondrocytes, and adipocytes [96].
MSC constitute an important part of the HSC niche. Moreover, human MSC transplanted subcutaneously form a humanized niche, able to support transplanted human HSC in mouse [97].
Several studies from Abraham group showed that HO-1 can also promote the MSC differentiation into osteoblasts and inhibit the MSC differentiation toward adipocytes while inhibiting HO-1 can promote adipogenesis [98,99,100]. However, other studies suggested that HO-1 overexpression has no effect on their differentiation potential [101, 102]. We and others have shown no differences in the differentiation potential between HO-1+/+ and HO-1−/− MSC [103, 104].
Yu et al. reported the negative correlation between HO-1 expression level in the bone marrow transplantation (BMT) recipients and acute-graft-versus-host disease (aGVHD) associated with allogeneic HSC transplantation [105]. The authors further used HO-1-overexpresing MSC to modulate the Th17/Treg ratio and prevent GVHD in mice [105].
HO-1 was shown to be responsible for the immunosuppressive effect of rat MSC on T cells [106]. HO-1 inhibition abrogated the protective effect of MSC on the heart allograft rejection [106]. Additionally, the overexpression of HO-1 improved the regeneration potential of allogenic MSC in porcine model of myocardial infarction after intracoronary infusion [107].
Altogether, being an essential part of the BM niche, MSC can influence HSC and hematopoiesis, but also through their immunomodulatory activity, they can regulate the function of the mature immune cells. In both cases, HO-1 is an important player (Fig. 3).
BM endothelial and CAR cells constitute the most important part of the HSC niche [108]. We observed that these two cell types highly express HO-1 [66]. The frequencies of EC and CAR cells were altered in HO-1−/− mice and they produced reduced amounts of TGF‐β1, SCF and SDF‐1α [66]—factors which are essential for maintaining the HSC quiescence [13, 109, 110]. Consequently, wild-type HSC transplanted into irradiated HO-1−/− mice lost their hematopoietic potential, whereas HO-1−/− HSC phenotype and functions were rescued when transplanted to the wild-type mice [66]. This suggests that the expression of HO-1 in HSC niche might be more important for sustaining the proper function of HSC than an expression of HO-1 in HSC themselves.
HO-1 as a regulator of innate and adaptive immunity
As indicated above, HO-1 activity affects the levels of many inflammatory mediators, but the current knowledge points to a more complex role of HO-1 in mounting both the innate and adaptive immune response at the cellular level [111].
Participation of HO-1 in the regulation of innate immunity was mainly investigated in the context of monocyte/macrophage lineage function. These studies were triggered by initial observations that HO-1−/− mice have elevated levels of MCP-1 (monocyte chemoattractant protein-1)—the proinflammatory factor regulating the response of monocytes and macrophages (reviewed in [112]). Further studies confirmed that lack of HO-1 causes the over-activated, proinflammatory phenotype of macrophages (reviewed in [111]). Otterbein et al. described the molecular mechanism that underlies this phenomenon. They showed that CO produced by HO-1 stimulates the production of IL-10 by macrophages [38]. Moreover, not only HO-1 activity stimulates the IL-10 production, but also IL-10 upregulates HO-1 expression [113]. This indicates the presence of a positive feedback mechanism and emphasizes the importance of HO-1 in anti-inflammatory function of IL-10.
The role of HO-1 in monocyte/macrophage lineage is not limited to the regulation of the function of mature macrophages. Recent study evidenced that HO-1 is involved in the maturation of the myeloid cells from hematopoietic stem and progenitor cells. The specific deletion of HO-1 in myeloid lineage (LysMCre/+Hmox1fl/fl) reduced the differentiation of myeloid progenitors toward macrophages [114]. It was shown that CO, produced by HO-1, stimulates the differentiation of myeloid progenitors to macrophages, increases CD14 on their surface and enhances sensitivity to M-CSF (macrophage colony-stimulating factor) stimulation [114].
The importance of HO-1 already in early differentiation steps of myeloid development was further confirmed by its role in myeloid-derived suppressor cells (MDSC)—a population representing the immature myeloid cells circulating in the peripheral blood [115]. HO-1 was crucial for the immunosuppressive function of MDSC population in the IL-10-dependent manner [116].
HO-1 deficiency in macrophages has an important consequences for the maintenance of organism homeostasis as well as for the resolution of pathological conditions. The latter was evidenced in a model of experimental autoimmune encephalomyelitis (EAE). The LysMCre/+Hmox1fl/fl mice developed more severe symptoms of EAE, what was correlated with exacerbated autoimmune T-cell response against myelin [117].
The connection of altered HO-1 expression in monocyte/macrophage lineage and autoimmune diseases are also supported by clinical observations. CD14+ monocytes from patients with autoimmunological systemic lupus erythematosus (SLE) have decreased HO-1 expression both at mRNA and protein level [118].
Studies performed by our group showed that lack of HO-1 disturbs also granulocyte numbers. HO-1−/− mice have more granulocytes in peripheral blood, what is connected with disordered maturation of granulocytes in the bone marrow. Myelocytes, the last dividing precursor stage in granulopoiesis, proliferate faster in HO-1−/− mice. Consistently, HO-1−/− myelocytes have a higher expression of C/EBPβ transcription factor, which drives the granulocyte differentiation [119].
Apart from important function in the regulation of innate immunity, HO-1 is also implicated in the regulation of adaptive immune response. First, HO-1 was shown to affect the maturation of dendritic cells (DC). HO-1 induction reduced the antigen presentation by DC and inhibited their pro-inflammatory function [120]. HO-1 in DC conserves IL-10 expression that is connected with anti-inflammatory DC phenotype [121]. The mechanism of anti-inflammatory role of HO-1 in DC was linked to CO-dependent reduction of TLR signaling [122].
As expected, the inhibition of DC maturation by HO-1 leads to reduced T-cell toxicity. This was shown in a model of transgenic mice that have autoreactive CD8+ T cells against insulin [122]. Such CD8+ T-cells induce diabetes after adoptive transfer only when previously immunized with DC. However, when DC were overexpressing HO-1, the CD8+ cells lost the ability to induce diabetes [122]. This confirms a crucial role of HO-1 in DC in regulation of the adaptive immunity.
Importantly, a lot of the studies concerning the influence of HO-1 on dendritic cells development/activity, including the recent ones, were done using CoPP (cobalt protoporphyrin IX) as an HO-1 activator. As shown already in 2008, some of the CoPP-induced effects on DC are HO-1 independent [123], so the conclusions from those experiments must be taken with caution.
Finally, HO-1 was proposed to regulate the function of suppressive T regulatory cells (Tregs). It was revealed that CD4+ CD25+ Tregs have a constant expression of HO-1 [124]. The pharmacological inhibition of HO-1 in Tregs diminished their suppressive function [125]. Nevertheless, the next studies raised doubts about HO-1 role in Tregs, as the Tregs isolated from HO-1−/− mice showed normal suppressive activity [126]. It was proposed, that it is HO-1 expression in DC that is required for Treg function, rather than intrinsic HO-1 in Tregs [127].
HO-1 and mobilization of bone marrow cells to peripheral blood
In steady-state conditions, mature blood cells are released from the bone marrow to the blood to sustain hematological homeostasis [128]. The bone marrow provides a barrier to limit egress of immature cells to peripheral blood and allows the circulation of only small numbers of hematopoietic stem cells [128]. In response to stress stimuli, such as inflammation or neutropenia, as well as in pathological conditions, such as developing tumor, hematopoiesis is accelerated and more cells are released from the bone marrow to the circulation [128] in a process called mobilization [129]. Several cytokines, such as SCF (stem cell factor), VEGF, IL-3, IL-6, GM-CSF and M-CSF regulate the mobilization of bone marrow cells, however, the most important are G-CSF and SDF-1α [129]. G-CSF increases the proliferation of the bone marrow myeloid progenitors as well as differentiation toward granulocytic lineage [130].
The influence of the HO-1 deficiency on the effect of G-CSF-induced mobilization is not clear. We observed that wild-type mice mobilize granulocytes better than HO-1−/− mice in response to G-CSF treatment [119], whereas the study by Ratajczak’s laboratory shows a rather opposite effect [131]. The possible reason for this discrepancy might be the genetic background—C57BL/6 × FVB [119] vs. 129 Sv × BALB/c [131] or model differences—the KO mice which we are using have elevated basal granulocyte numbers compared to wild type littermates [48] and such effect was not observed in the study by Ratajczak’s group [131].
Two papers from Ratajczak’s laboratory show the modulation of mobilizing factors effect by pharmacological modulation of HO-1 activity. Injection of HO-1 inhibitor (SnPP) together with standard mobilizing factors, G-CSF or plerixafor, potentiated their mobilizing effect [131]. Oppositely, treatment of mice with HO-1 inducer (CoPP) lead to the decreased mobilization of neutrophils from the bone marrow to lungs in response to LPS [132].
Another study from the same group showed that ex vivo incubation of BM-MNCs with HO-1 activity inhibitor, SnPP, resulted in their increased homing to BM after transplantation to the irradiated mice [133]. However, the experiments with protoporphyrins must be evaluated with caution, as CoPP given alone increases the expression of endogenous G-CSF and induces mobilization of granulocytes and HSP [134]. Using HO-1 deficient mice we showed that this effect is independent of HO-1 [134].
HO-1 independent effects of protoporphyrins
CoPP is a known HO-1 inducer, used in many in vitro and in vivo studies and usually the effects of CoPP have been attributed to HO-1 activity. CoPP activates HO-1 expression by destabilization of Bach1 repressor and stabilization of the Nrf2 transcription factor [135]. Although CoPP is not found in normal conditions in the organism, it may be formed in vivo after CoCl2 injection [136]. Metalloporphyrins in which iron is replaced by other metals, are able to induce HO-1 expression at mRNA and protein level, just as heme does [137, 138]. However, at the same time non-heme porphyrins are the competitive inhibitors of HO-1 [137,138,139]. The increase in HO-1 expression induced by CoPP prevails over the transient inhibitory effect of CoPP on HO-1 activity [137]. Oppositely, SnPP inhibits HO-1 activity more strongly than CoPP and at the same time less potently upregulates HO-1 expression [137]. Overall, concerning the final effect, CoPP is used as HO-1 inducer and SnPP as HO-1 inhibitor, but one has to remember that both can exert similar activities. The level of HO-1 induction by metalloporphyrins as well as its duration depend on the metal [137, 139]. CoPP is a rapid inducer of Hmox1 mRNA, but the maximal induction of HO-1 activity occurs at the later time than after stimulation with heme and lasts longer [139, 140].
The possibility that porphyrins may have several activities that are independent of their HO-1 modulatory function was already suggested by us and others. Our group showed that two HO-1 inhibitors (SnPP and ZnPP) have opposite effects on nitric oxide production by iNOS, after the stimulation of cells with IL-1β or LPS [141]. Blumenthal and coworkers reported that both CoPP and SnPP can directly inhibit caspase-3 and -8 activity, independently of HO-1 [138]. Both of these caspases were shown to play a role in hematopoiesis [142]. Inhibition of caspase-3 and -8 decreases human neutrophil apoptosis [143].
Different metalloporphyrins may also have different effects on the bone marrow cells—zinc porphyrins (ZnPP and ZnMP) where shown to inhibit hematopoiesis in vitro, whereas other HO-1 inbibitors—tin porphyrins had no effect [144].
Two other studies indicate the HO-1-independent modulation of immune reaction by CoPP. One of them shows that CoPP inhibited LPS-stimulated activation of inducible nitric oxide synthase (iNOS) in RAW264.7 macrophages by blocking JNK phosphorylation [145]. Silencing of HO-1 with siRNA did not affect iNOS inhibition by CoPP [145]. Similarly, cyclooxygenase-2 (COX-2) upregulation by CoPP in microglia was proved to be HO-1-independent by the use of HO-1 siRNA [146].
Those findings indicate that the conclusions about the HO-1 influence on a given process based on protoporphyrin treatment need to be confirmed using genetic models.
Conclusions
HO-1 plays an important role in the maintenance of the hematopoiesis, ensures the proper function of the HSC niche and regulates the differentiation of progenitors to mature blood cells. HO-1 affects the immune system by directly influencing the immune cells, but also by influencing the immunomodulatory function of other cell types involved in immune response, such as endothelial or mesenchymal stromal cells.
References
Seita J, Weissman IL (2010) Hematopoietic stem cell: self-renewal versus differentiation. WIREs Syst Biol Med 2:640–653. https://doi.org/10.1002/wsbm.86
Morrison SJ, Uchida N, Weissman IL (1995) The biology of hematopoietic stem cells. Annu Rev Cell Dev Biol 11:35–71. https://doi.org/10.1146/annurev.cb.11.110195.000343
Spangrude GJ, Heimfeld S, Weissman IL (1988) Purification and characterization of mouse hematopoietic stem cells. Science 241:58–62. https://doi.org/10.1126/science.2898810
Morrison SJ, Weissman IL (1994) The long-term repopulating subset of hematopoietic stem cells is deterministic and lsolatable by phenotype. Stem Cells. https://doi.org/10.1016/1074-7613(94)90037-x
Akashi K, Traver D, Miyamoto T, Weissman IL (2000) A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature 404:193–197. https://doi.org/10.1038/35004599
Debili N, Coulombel L, Croisille L et al (1996) Characterization of a bipotent erythro-megakaryocytic progenitor in human bone marrow. Blood 88:1284–1296
Pronk CJH, Rossi DJ, Månsson R et al (2007) Elucidation of the phenotypic, functional, and molecular topography of a myeloerythroid progenitor cell hierarchy. Cell Stem Cell 1:428–442. https://doi.org/10.1016/j.stem.2007.07.005
Yamamoto R, Morita Y, Ooehara J et al (2013) Clonal analysis unveils self-renewing lineage-restricted progenitors generated directly from hematopoietic stem cells. Cell 154:1112–1126. https://doi.org/10.1016/j.cell.2013.08.007
Notta F, Zandi S, Takayama N et al (2016) Distinct routes of lineage development reshape the human blood hierarchy across ontogeny. Science. https://doi.org/10.1126/science.aab2116
Boulais PE, Frenette PS (2015) Making sense of hematopoietic stem cell niches. Blood 125:2621–2629. https://doi.org/10.1182/blood-2014-09-570192
Schofield R (1978) The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells 4:7–25
Whitlock CA, Tidmarsh GF, Muller-Sieburg C, Weissman IL (1987) Bone marrow stromal cell lines with lymphopoietic activity express high levels of a pre-B neoplasia-associated molecule. Cell 48:1009–1021. https://doi.org/10.1016/0092-8674(87)90709-4
Sugiyama T, Kohara H, Noda M, Nagasawa T (2006) Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity 25:977–988. https://doi.org/10.1016/j.immuni.2006.10.016
Zhou BO, Yue R, Murphy MM et al (2014) Leptin-receptor-expressing mesenchymal stromal cells represent the main source of bone formed by adult bone marrow. Cell Stem Cell 15:154–168. https://doi.org/10.1016/j.stem.2014.06.008
Zhang J, Niu C, Ye L et al (2003) Identification of the haematopoietic stem cell niche and control of the niche size. Nature 425:836–841. https://doi.org/10.1038/nature02041
Calvi LM, Adams GB, Weibrecht KW et al (2003) Osteoblastic cells regulate the haematopoietic stem cell niche. Nature 425:841–846. https://doi.org/10.1038/nature02040
Kiel MJ, Yilmaz OH, Iwashita T et al (2005) SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell 121:1109–1121. https://doi.org/10.1016/j.cell.2005.05.026
Arinobu Y, Mizuno S, Chong Y et al (2007) Reciprocal activation of GATA-1 and PU.1 marks initial specification of hematopoietic stem cells into myeloerythroid and myelolymphoid lineages. Cell Stem Cell 1:416–427. https://doi.org/10.1016/j.stem.2007.07.004
Szade K, Gulati GS, Chan CKF et al (2018) Where hematopoietic stem cells live: the bone marrow niche. Antioxid Redox Signal 29:191–204. https://doi.org/10.1089/ars.2017.7419
Nakamura-Ishizu A, Takubo K, Kobayashi H et al (2015) CLEC-2 in megakaryocytes is critical for maintenance of hematopoietic stem cells in the bone marrow. J Exp Med 212:2133–2146. https://doi.org/10.1084/jem.20150057
Greenbaum A, Hsu Y-MS, Day RB et al (2013) CXCL12 in early mesenchymal progenitors is required for haematopoietic stem-cell maintenance. Nature 495:227–230. https://doi.org/10.1038/nature11926
Omatsu Y, Sugiyama T, Kohara H et al (2010) The essential functions of adipo-osteogenic progenitors as the hematopoietic stem and progenitor cell niche. Immunity 33:387–399. https://doi.org/10.1016/j.immuni.2010.08.017
Bruns I, Lucas D, Pinho S et al (2014) Megakaryocytes regulate hematopoietic stem cell quiescence through CXCL4 secretion. Nat Med 20:1315–1320. https://doi.org/10.1038/nm.3707
Zhao M, Perry JM, Marshall H et al (2014) Megakaryocytes maintain homeostatic quiescence and promote post-injury regeneration of hematopoietic stem cells. Nat Med 20:1321–1326. https://doi.org/10.1038/nm.3706
Nakamura-Ishizu A, Takubo K, Fujioka M, Suda T (2014) Megakaryocytes are essential for HSC quiescence through the production of thrombopoietin. Biochem Biophys Res Commun 454:353–357. https://doi.org/10.1016/j.bbrc.2014.10.095
Till JE, McCULLOCH EA (1961) A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Radiat Res 14:213–222
Becker AJ, McCULLOCH EA, Till JE (1963) Cytological demonstration of the clonal nature of spleen colonies derived from transplanted mouse marrow cells. Nature 197:452–454. https://doi.org/10.1038/197452a0
Baryawno N, Severe N, Scadden DT (2017) Hematopoiesis: reconciling historic controversies about the niche. Cell Stem Cell 20:590–592. https://doi.org/10.1016/j.stem.2017.03.025
Tenhunen R, Marver HS, Schmid R (1968) The enzymatic conversion of heme to bilirubin by microsomal heme oxygenase. Proc Natl Acad Sci U S A 61:748–755
Tenhunen R, Ross ME, Marver HS, Schmid R (1970) Reduced nicotinamide-adenine dinucleotide phosphate dependent biliverdin reductase: partial purification and characterization. Biochemistry 9:298–303
Ryter SW, Alam J, Choi AMK (2006) Heme oxygenase-1/carbon monoxide: from basic science to therapeutic applications. Physiol Rev 86:583–650. https://doi.org/10.1152/physrev.00011.2005
Maines MD, Trakshel GM, Kutty RK (1986) Characterization of two constitutive forms of rat liver microsomal heme oxygenase. Only one molecular species of the enzyme is inducible. J Biol Chem 261:411–419
Morse D, Choi AMK (2002) Heme oxygenase-1: the “emerging molecule” has arrived. Am J Respir Cell Mol Biol 27:8–16. https://doi.org/10.1165/ajrcmb.27.1.4862
Ewing JF, Maines MD (1995) Distribution of constitutive (HO-2) and heat-inducible (HO-1) heme oxygenase isozymes in rat testes: HO-2 displays stage-specific expression in germ cells. Endocrinology 136:2294–2302. https://doi.org/10.1210/endo.136.5.7720678
Sun Y, Rotenberg MO, Maines MD (1990) Developmental expression of heme oxygenase isozymes in rat brain. Two HO-2 mRNAs are detected. J Biol Chem 265:8212–8217
Jeney V, Balla J, Yachie A et al (2002) Pro-oxidant and cytotoxic effects of circulating heme. Blood 100:879–887. https://doi.org/10.1182/blood.v100.3.879
Bozza MT, Jeney V (2020) Pro-inflammatory actions of heme and other hemoglobin-derived DAMPs. Front Immunol. https://doi.org/10.3389/fimmu.2020.01323
Otterbein LE, Bach FH, Alam J et al (2000) Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat Med 6:422–428. https://doi.org/10.1038/74680
Nakahira K, Kim HP, Geng XH et al (2006) Carbon monoxide differentially inhibits TLR signaling pathways by regulating ROS-induced trafficking of TLRs to lipid rafts. J Exp Med 203:2377–2389. https://doi.org/10.1084/jem.20060845
Zhang X, Shan P, Alam J et al (2003) Carbon monoxide modulates Fas/Fas ligand, caspases, and Bcl-2 family proteins via the p38alpha mitogen-activated protein kinase pathway during ischemia-reperfusion lung injury. J Biol Chem 278:22061–22070. https://doi.org/10.1074/jbc.M301858200
Wegiel B, Otterbein LE (2012) Go green: the anti-inflammatory effects of biliverdin reductase. Front Pharmacol. https://doi.org/10.3389/fphar.2012.00047
Jansen T, Daiber A (2012) Direct antioxidant properties of bilirubin and biliverdin. Is there a role for biliverdin reductase? Front Pharmacol. https://doi.org/10.3389/fphar.2012.00030
Mazzone GL, Rigato I, Ostrow JD et al (2009) Bilirubin inhibits the TNFα-related induction of three endothelial adhesion molecules. Biochem Biophys Res Commun 386:338–344. https://doi.org/10.1016/j.bbrc.2009.06.029
Vogel ME, Zucker SD (2016) Bilirubin acts as an endogenous regulator of inflammation by disrupting adhesion molecule-mediated leukocyte migration. Inflamm Cell Signal 3(1):e1178
Balla G, Jacob HS, Balla J et al (1992) Ferritin: a cytoprotective antioxidant strategem of endothelium. J Biol Chem 267:18148–18153
Berberat PO, Katori M, Kaczmarek E et al (2003) Heavy chain ferritin acts as an anti-apoptotic gene that protects livers from ischemia-reperfusion injury. FASEB J 17:1724–1726. https://doi.org/10.1096/fj.03-0229fje
Ghoreschi K, Brück J, Kellerer C et al (2011) Fumarates improve psoriasis and multiple sclerosis by inducing type II dendritic cells. J Exp Med 208:2291–2303. https://doi.org/10.1084/jem.20100977
Poss KD, Tonegawa S (1997) Heme oxygenase 1 is required for mammalian iron reutilization. Proc Natl Acad Sci 94:10919–10924. https://doi.org/10.1073/pnas.94.20.10919
Yachie A, Niida Y, Wada T et al (1999) Oxidative stress causes enhanced endothelial cell injury in human heme oxygenase-1 deficiency. J Clin Invest 103:129–135. https://doi.org/10.1172/JCI4165
Radhakrishnan N, Yadav SP, Sachdeva A et al (2011) An interesting tetrad of asplenia, inflammation, hemolysis, and nephritis. Pediatr Hematol Oncol 28:723–726. https://doi.org/10.3109/08880018.2011.613979
Radhakrishnan N, Yadav SP, Sachdeva A et al (2011) Human heme oxygenase-1 deficiency presenting with hemolysis, nephritis, and asplenia. J Pediatr Hematol Oncol 33:74–78. https://doi.org/10.1097/MPH.0b013e3181fd2aae
Gupta A, Akihiro Y, Saxena AK et al (2016) Haem oxygenase-1 deficiency: a mimicker of childhood vasculitis. Scand J Rheumatol 45:165–166. https://doi.org/10.3109/03009742.2015.1092583
Yachie A (2019) Heme oxygenase-1 deficiency. In: Oohashi T, Tsukahara H, Ramirez F et al (eds) Human pathobiochemistry: from clinical studies to molecular mechanisms. Springer, Singapore, pp 67–79
Tahghighi F, Parvaneh N, Ziaee V (2019) Post-mortem diagnosis of heme oxygenase-1 deficiency by whole exome sequencing in an iranian child. Int J Mol Cell Med 8:300–307. https://doi.org/10.22088/IJMCM.BUMS.8.4.300
Kovtunovych G, Eckhaus MA, Ghosh MC et al (2010) Dysfunction of the heme recycling system in heme oxygenase 1–deficient mice: effects on macrophage viability and tissue iron distribution. Blood 116:6054–6062. https://doi.org/10.1182/blood-2010-03-272138
Kovtunovych G, Ghosh MC, Ollivierre W et al (2014) Wild-type macrophages reverse disease in heme oxygenase 1-deficient mice. Blood 124:1522–1530. https://doi.org/10.1182/blood-2014-02-554162
Yadav SP, Thakkar D, Kohli S et al (2018) Human heme-oxygenase-1 deficiency treated successfully by matched sibling donor allogeneic stem cell transplant. Biol Blood Marrow Transplant 24:S443. https://doi.org/10.1016/j.bbmt.2017.12.537
Kimpara T, Takeda A, Watanabe K et al (1997) Microsatellite polymorphism in the human heme oxygenase-1 gene promoter and its application in association studies with Alzheimer and Parkinson disease. Hum Genet 100:145–147. https://doi.org/10.1007/s004390050480
Exner M, Minar E, Wagner O, Schillinger M (2004) The role of heme oxygenase-1 promoter polymorphisms in human disease. Free Radic Biol Med 37:1097–1104. https://doi.org/10.1016/j.freeradbiomed.2004.07.008
Taha H, Skrzypek K, Guevara I et al (2010) Role of heme oxygenase-1 in human endothelial cells - lesson from the promoter allelic variants. Arterioscler Thromb Vasc Biol 30:1634–1641. https://doi.org/10.1161/ATVBAHA.110.207316
Bao W, Song F, Li X et al (2010) association between heme oxygenase-1 gene promoter polymorphisms and type 2 diabetes mellitus: a HuGE review and meta-analysis. Am J Epidemiol 172:631–636. https://doi.org/10.1093/aje/kwq162
Agúndez JAG, García-Martín E, Martínez C et al (2016) Heme oxygenase-1 and 2 common genetic variants and risk for multiple sclerosis. Sci Rep. https://doi.org/10.1038/srep20830
Bukowska-Strakova K, Włodek J, Pitera E et al (2021) Role of HMOX1 promoter genetic variants in chemoresistance and chemotherapy induced neutropenia in children with acute lymphoblastic leukemia. Int J Mol Sci. https://doi.org/10.3390/ijms22030988
Ayer A, Zarjou A, Agarwal A, Stocker R (2016) Heme oxygenases in cardiovascular health and disease. Physiol Rev 96:1449–1508. https://doi.org/10.1152/physrev.00003.2016
Lüblinghoff N, Winkler K, Winkelmann BR et al (2009) Genetic variants of the promoter of the heme oxygenase-1 gene and their influence on cardiovascular disease (The Ludwigshafen Risk and Cardiovascular Health Study). BMC Med Genet 10:36. https://doi.org/10.1186/1471-2350-10-36
Szade K, Zukowska M, Szade A et al (2020) Heme oxygenase-1 deficiency triggers exhaustion of hematopoietic stem cells. EMBO Rep. https://doi.org/10.15252/embr.201947895
So AY-L, Garcia-Flores Y, Minisandram A et al (2012) Regulation of APC development, immune response, and autoimmunity by Bach1/HO-1 pathway in mice. Blood 120:2428–2437. https://doi.org/10.1182/blood-2012-04-426247
Oyake T, Itoh K, Motohashi H et al (1996) Bach proteins belong to a novel family of BTB-basic leucine zipper transcription factors that interact with MafK and regulate transcription through the NF-E2 site. Mol Cell Biol 16:6083–6095
Suzuki H, Tashiro S, Hira S et al (2004) Heme regulates gene expression by triggering Crm1-dependent nuclear export of Bach1. EMBO J 23:2544–2553. https://doi.org/10.1038/sj.emboj.7600248
Zenke-Kawasaki Y, Dohi Y, Katoh Y et al (2007) Heme induces ubiquitination and degradation of the transcription factor bach1. Mol Cell Biol 27:6962–6971. https://doi.org/10.1128/MCB.02415-06
Itoh-Nakadai A, Hikota R, Muto A et al (2014) The transcription repressors Bach2 and Bach1 promote B cell development by repressing the myeloid program. Nat Immunol 15:1171–1180. https://doi.org/10.1038/ni.3024
Watanabe-Matsui M, Muto A, Matsui T et al (2011) Heme regulates B-cell differentiation, antibody class switch, and heme oxygenase-1 expression in B cells as a ligand of Bach2. Blood 117:5438–5448. https://doi.org/10.1182/blood-2010-07-296483
Cao Y-A, Wagers AJ, Karsunky H et al (2008) Heme oxygenase-1 deficiency leads to disrupted response to acute stress in stem cells and progenitors. Blood 112:4494–4502. https://doi.org/10.1182/blood-2007-12-127621
Abraham NG, Kappas A (2008) Pharmacological and clinical aspects of heme oxygenase. Pharmacol Rev 60:79–127. https://doi.org/10.1124/pr.107.07104
Ibrahim NG, Lutton JD, Levere RD (1982) The role of haem biosynthetic and degradative enzymes in erythroid colony development: the effect of haemin. Br J Haematol 50:17–28. https://doi.org/10.1111/j.1365-2141.1982.tb01886.x
Abraham NG (1991) Molecular regulation—biological role of heme in hematopoiesis. Blood Rev 5:19–28. https://doi.org/10.1016/0268-960X(91)90004-V
Alves LR, Costa ES, Sorgine MHF et al (2011) Heme-Oxygenases during Erythropoiesis in K562 and Human Bone Marrow Cells. PLoS ONE. https://doi.org/10.1371/journal.pone.0021358
Garcia-Santos D, Schranzhofer M, Horvathova M et al (2014) Heme oxygenase 1 is expressed in murine erythroid cells where it controls the level of regulatory heme. Blood 123:2269–2277. https://doi.org/10.1182/blood-2013-04-496760
Fraser ST, Midwinter RG, Coupland LA et al (2015) Heme oxygenase-1 deficiency alters erythroblastic island formation, steady-state erythropoiesis and red blood cell lifespan in mice. Haematologica 100:601–610. https://doi.org/10.3324/haematol.2014.116368
Chasis JA, Mohandas N (2008) Erythroblastic islands: niches for erythropoiesis. Blood 112:470–478. https://doi.org/10.1182/blood-2008-03-077883
Toobiak S, Shaklai M, Shaklai N (2012) Carbon Monoxide Induced Erythroid Differentiation of K562 Cells Mimics the Central Macrophage Milieu in Erythroblastic Islands. PLoS ONE. https://doi.org/10.1371/journal.pone.0033940
Cao Y-A, Kusy S, Luong R et al (2011) Heme Oxygenase-1 Deletion Affects Stress Erythropoiesis. PLoS ONE 6:e20634. https://doi.org/10.1371/journal.pone.0020634
Kato H, Itoh-Nakadai A, Matsumoto M et al (2018) Infection perturbs Bach2- and Bach1-dependent erythroid lineage ‘choice’ to cause anemia. Nat Immunol 19:1059–1070. https://doi.org/10.1038/s41590-018-0202-3
Kato H, Igarashi K (2019) To be red or white: lineage commitment and maintenance of the hematopoietic system by the “inner myeloid.” Haematologica 104:1919–1927. https://doi.org/10.3324/haematol.2019.216861
Loboda A, Jazwa A, Grochot-Przeczek A et al (2008) Heme oxygenase-1 and the vascular bed: from molecular mechanisms to therapeutic opportunities. Antioxid Redox Signal 10:1767–1812. https://doi.org/10.1089/ars.2008.2043
Calay D, Mason JC (2013) The Multifunctional Role and Therapeutic Potential of HO-1 in the Vascular Endothelium. Antioxid Redox Signal 20:1789–1809. https://doi.org/10.1089/ars.2013.5659
Kim Y-M, Pae H-O, Park JE et al (2011) Heme oxygenase in the regulation of vascular biology: from molecular mechanisms to therapeutic opportunities. Antioxid Redox Signal 14:137–167. https://doi.org/10.1089/ars.2010.3153
Soares MP, Lin Y, Anrather J et al (1998) Expression of heme oxygenase-1 can determine cardiac xenograft survival. Nat Med 4:1073–1077. https://doi.org/10.1038/2063
Brouard S, Otterbein LE, Anrather J et al (2000) Carbon Monoxide Generated by Heme Oxygenase 1 Suppresses Endothelial Cell Apoptosis. J Exp Med 192:1015–1026. https://doi.org/10.1084/jem.192.7.1015
Deramaudt BMJM, Braunstein S, Remy P, Abraham NG (1998) Gene transfer of human heme oxygenase into coronary endothelial cells potentially promotes angiogenesis. J Cell Biochem 68:121–127. https://doi.org/10.1002/(SICI)1097-4644(19980101)68:1%3c121::AID-JCB12%3e3.0.CO;2-K
Dulak J, Deshane J, Jozkowicz A, Agarwal A (2008) Heme oxygenase-1 and carbon monoxide in vascular pathobiology: focus on angiogenesis. Circulation 117:231–241. https://doi.org/10.1161/CIRCULATIONAHA.107.698316
Soares MP, Seldon MP, Gregoire IP et al (1950) (2004) Heme oxygenase-1 modulates the expression of adhesion molecules associated with endothelial cell activation. J Immunol Baltim Md 172:3553–3563. https://doi.org/10.4049/jimmunol.172.6.3553
Seldon MP, Silva G, Pejanovic N et al (2007) Heme oxygenase-1 inhibits the expression of adhesion molecules associated with endothelial cell activation via inhibition of Nf-κB rela phosphorylation at serine 276. J Immunol 179:7840–7851. https://doi.org/10.4049/jimmunol.179.11.7840
Caplan AI (2017) Mesenchymal stem cells: time to change the name! Stem Cells Transl Med 6:1445–1451. https://doi.org/10.1002/sctm.17-0051
Kern S, Eichler H, Stoeve J et al (2006) Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells Dayt Ohio 24:1294–1301. https://doi.org/10.1634/stemcells.2005-0342
Kolf CM, Cho E, Tuan RS (2007) Mesenchymal stromal cells. Biology of adult mesenchymal stem cells: regulation of niche, self-renewal and differentiation. Arthritis Res Ther 9:204. https://doi.org/10.1186/ar2116
Reinisch A, Thomas D, Corces MR et al (2016) A humanized ossicle-niche xenotransplantation model enables improved human leukemic engraftment. Nat Med 22:812–821. https://doi.org/10.1038/nm.4103
Barbagallo I, Vanella A, Peterson SJ et al (2010) Overexpression of heme oxygenase-1 increases human osteoblast stem cell differentiation. J Bone Miner Metab 28:276–288. https://doi.org/10.1007/s00774-009-0134-y
Vanella L, Kim DH, Asprinio D et al (2010) HO-1 expression increases mesenchymal stem cell-derived osteoblast but decreases adipocyte lineage. Bone 46:236. https://doi.org/10.1016/j.bone.2009.10.012
Vanella L, Sanford C, Kim DH et al (2012) Oxidative stress and heme oxygenase-1 regulated human mesenchymal stem cells differentiation. Int J Hypertens 2012:890671. https://doi.org/10.1155/2012/890671
Zhou H, Ramiya VK, Visner GA (2006) Bone marrow stem cells as a vehicle for delivery of heme oxygenase-1 gene. Stem Cells Dev 15:79–86. https://doi.org/10.1089/scd.2006.15.79
Hamedi-Asl P, Halabian R, Bahmani P et al (2012) Adenovirus-mediated expression of the HO-1 protein within MSCs decreased cytotoxicity and inhibited apoptosis induced by oxidative stresses. Cell Stress Chaperones 17:181–190. https://doi.org/10.1007/s12192-011-0298-y
Zarjou A, Kim J, Traylor AM et al (2011) Paracrine effects of mesenchymal stem cells in cisplatin-induced renal injury require heme oxygenase-1. Am J Physiol Ren Physiol 300:F254–F262. https://doi.org/10.1152/ajprenal.00594.2010
Nowak WN, Taha H, Kachamakova-Trojanowska N et al (2018) Murine bone marrow mesenchymal stromal cells respond efficiently to oxidative stress despite the low level of heme oxygenases 1 and 2. Antioxid Redox Signal 29:111–127. https://doi.org/10.1089/ars.2017.7097
Yu M, Wang J, Fang Q et al (2016) High expression of heme oxygenase-1 in target organs may attenuate acute graft-versus-host disease through regulation of immune balance of TH17/Treg. Transpl Immunol 37:10–17. https://doi.org/10.1016/j.trim.2016.05.002
Chabannes D, Hill M, Merieau E et al (2007) A role for heme oxygenase-1 in the immunosuppressive effect of adult rat and human mesenchymal stem cells. Blood 110:3691–3694. https://doi.org/10.1182/blood-2007-02-075481
Wojakowski W, Tendera M, Cybulski W et al (2012) Effects of intracoronary delivery of allogenic bone marrow-derived stem cells expressing heme oxygenase-1 on myocardial reperfusion injury. Thromb Haemost 108:464–475. https://doi.org/10.1160/TH12-05-0303
Morrison SJ, Scadden DT (2014) The bone marrow niche for haematopoietic stem cells. Nature 505:327–334. https://doi.org/10.1038/nature12984
Sitnicka E, Ruscetti FW, Priestley GV et al (1996) Transforming growth factor beta 1 directly and reversibly inhibits the initial cell divisions of long-term repopulating hematopoietic stem cells. Blood 88:82–88
Ding L, Saunders TL, Enikolopov G, Morrison SJ (2012) Endothelial and perivascular cells maintain haematopoietic stem cells. Nature 481:457–462. https://doi.org/10.1038/nature10783
Blancou P, Tardif V, Simon T et al (2011) Immunoregulatory properties of heme oxygenase-1. Methods Mol Biol Clifton NJ 677:247–268. https://doi.org/10.1007/978-1-60761-869-0_18
Deshmane SL, Kremlev S, Amini S, Sawaya BE (2009) Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interferon Cytokine Res 29:313–326. https://doi.org/10.1089/jir.2008.0027
Lee T-S, Chau L-Y (2002) Heme oxygenase-1 mediates the anti-inflammatory effect of interleukin-10 in mice. Nat Med 8:240–246. https://doi.org/10.1038/nm0302-240
Wegiel B, Hedblom A, Li M et al (2014) Heme oxygenase-1 derived carbon monoxide permits maturation of myeloid cells. Cell Death Dis 5:e1139. https://doi.org/10.1038/cddis.2014.97
Condamine T, Gabrilovich DI (2011) Molecular mechanisms regulating myeloid-derived suppressor cell differentiation and function. Trends Immunol 32:19–25. https://doi.org/10.1016/j.it.2010.10.002
De Wilde V, Van Rompaey N, Hill M et al (2009) Endotoxin-induced myeloid-derived suppressor cells inhibit alloimmune responses via heme oxygenase-1. Am J Transplant 9:2034–2047. https://doi.org/10.1111/j.1600-6143.2009.02757.x
Tzima S, Victoratos P, Kranidioti K et al (2009) Myeloid heme oxygenase–1 regulates innate immunity and autoimmunity by modulating IFN-β production. J Exp Med 206:1167–1179. https://doi.org/10.1084/jem.20081582
Herrada AA, Llanos C, Mackern-Oberti JP et al (2012) Haem oxygenase 1 expression is altered in monocytes from patients with systemic lupus erythematosus. Immunology 136:414–424. https://doi.org/10.1111/j.1365-2567.2012.03598.x
Bukowska-Strakova K, Ciesla M, Szade K et al (2017) Heme oxygenase 1 affects granulopoiesis in mice through control of myelocyte proliferation. Immunobiology 222:506–517. https://doi.org/10.1016/j.imbio.2016.10.018
Chora AA, Fontoura P, Cunha A et al (2007) Heme oxygenase-1 and carbon monoxide suppress autoimmune neuroinflammation. J Clin Invest 117:438–447. https://doi.org/10.1172/JCI28844
Chauveau C, Rémy S, Royer PJ et al (2005) Heme oxygenase-1 expression inhibits dendritic cell maturation and proinflammatory function but conserves IL-10 expression. Blood 106:1694–1702. https://doi.org/10.1182/blood-2005-02-0494
Rémy S, Blancou P, Tesson L et al (1950) (2009) Carbon monoxide inhibits TLR-induced dendritic cell immunogenicity. J Immunol Baltim Md 182:1877–1884. https://doi.org/10.4049/jimmunol.0802436
Mashreghi M-F, Klemz R, Knosalla IS et al (2008) Inhibition of dendritic cell maturation and function is independent of heme oxygenase 1 but requires the activation of STAT3. J Immunol 180:7919–7930. https://doi.org/10.4049/jimmunol.180.12.7919
Pae H-O, Oh G-S, Choi B-M et al (2003) Differential expressions of heme oxygenase-1 gene in CD25- and CD25+ subsets of human CD4+ T cells. Biochem Biophys Res Commun 306:701–705
Choi B-M, Pae H-O, Jeong Y-R et al (2005) Critical role of heme oxygenase-1 in Foxp3-mediated immune suppression. Biochem Biophys Res Commun 327:1066–1071. https://doi.org/10.1016/j.bbrc.2004.12.106
Zelenay S, Chora A, Soares MP, Demengeot J (2007) Heme oxygenase-1 is not required for mouse regulatory T cell development and function. Int Immunol 19:11–18. https://doi.org/10.1093/intimm/dxl116
George JF, Braun A, Brusko TM et al (2008) Suppression by CD4+CD25+ regulatory T cells is dependent on expression of heme oxygenase-1 in antigen-presenting cells. Am J Pathol 173:154–160. https://doi.org/10.2353/ajpath.2008.070963
Weiss L, Geduldig U (1991) Barrier cells: stromal regulation of hematopoiesis and blood cell release in normal and stressed murine bone marrow. Blood 78:975–990. https://doi.org/10.1182/blood.V78.4.975.975
Lapid K, Glait-Santar C, Gur-Cohen S et al (2008) Egress and mobilization of hematopoietic stem and progenitor cells: a dynamic multi-facet process. StemBook. https://doi.org/10.3824/stembook.1.39.1
Beekman R, Touw IP (2010) G-CSF and its receptor in myeloid malignancy. Blood 115:5131–5136. https://doi.org/10.1182/blood-2010-01-234120
Wysoczynski M, Ratajczak J, Pedziwiatr D et al (2015) Identification of heme oxygenase 1 (HO-1) as a novel negative regulator of mobilization of hematopoietic stem/progenitor cells. Stem Cell Rev Rep 11:110–118. https://doi.org/10.1007/s12015-014-9547-7
Konrad FM, Braun S, Ngamsri K-C et al (2014) Heme oxygenase-1 attenuates acute pulmonary inflammation by decreasing the release of segmented neutrophils from the bone marrow. Am J Physiol Lung Cell Mol Physiol 307:L707-717. https://doi.org/10.1152/ajplung.00145.2014
Adamiak M, Iv JBM, Zhao J et al (2015) Downregulation of heme oxygenase 1 (HO-1) activity in hematopoietic cells enhances their engraftment after transplantation. Cell Transplant. https://doi.org/10.3727/096368915X688957
Szade A, Szade K, Nowak WN et al (2019) Cobalt protoporphyrin IX increases endogenous G-CSF and mobilizes HSC and granulocytes to the blood. EMBO Mol Med 11:e09571. https://doi.org/10.15252/emmm.201809571
Shan Y, Lambrecht RW, Donohue SE, Bonkovsky HL (2006) Role of Bach1 and Nrf2 in up-regulation of the heme oxygenase-1 gene by cobalt protoporphyrin. FASEB J Off Publ Fed Am Soc Exp Biol 20:2651–2653. https://doi.org/10.1096/fj.06-6346fje
Sinclair P, Gibbs AH, Sinclair JF, de Matteis F (1979) Formation of cobalt protoporphyrin in the liver of rats. A mechanism for the inhibition of liver haem biosynthesis by inorganic cobalt. Biochem J 178:529–538
Sardana MK, Kappas A (1987) Dual control mechanism for heme oxygenase: tin(IV)-protoporphyrin potently inhibits enzyme activity while markedly increasing content of enzyme protein in liver. Proc Natl Acad Sci 84:2464–2468
Blumenthal SB, Kiemer AK, Tiegs G et al (2005) Metalloporphyrins inactivate caspase-3 and -8. FASEB J 19:1272–1279. https://doi.org/10.1096/fj.04-3259com
Drummond GS, Baum J, Greenberg M et al (2019) HO-1 overexpression and underexpression: Clinical implications. Arch Biochem Biophys 673:108073. https://doi.org/10.1016/j.abb.2019.108073
Drummond GS, Kappas A (1982) The cytochrome P-450-depleted animal: an experimental model for in vivo studies in chemical biology. Proc Natl Acad Sci 79:2384–2388. https://doi.org/10.1073/pnas.79.7.2384
Józkowicz A, Dulak J (2003) Effects of protoporphyrins on production of nitric oxide and expression of vascular endothelial growth factor in vascular smooth muscle cells and macrophages. Acta Biochim Pol 50:69–79
Droin N, Cathelin S, Jacquel A et al (2008) A role for caspases in the differentiation of erythroid cells and macrophages. Biochimie 90:416–422. https://doi.org/10.1016/j.biochi.2007.08.007
Alvarado-Kristensson M, Melander F, Leandersson K et al (2004) p38-MAPK signals survival by phosphorylation of caspase-8 and caspase-3 in human neutrophils. J Exp Med 199:449–458. https://doi.org/10.1084/jem.20031771
Lutton JD, Abraham NG, Drummond GS et al (1997) Zinc porphyrins: potent inhibitors of hematopoieses in animal and human bone marrow. Proc Natl Acad Sci U S A 94:1432–1436
Lin H-Y, Shen S-C, Lin C-W et al (2009) Cobalt protoporphyrin inhibition of lipopolysaccharide or lipoteichoic acid-induced nitric oxide production via blocking c-Jun N-terminal kinase activation and nitric oxide enzyme activity. Chem Biol Interact 180:202–210. https://doi.org/10.1016/j.cbi.2009.01.004
Lin H-Y, Tsai C-H, Lin C et al (2015) Cobalt protoporphyrin upregulates cyclooxygenase-2 expression through a heme oxygenase-independent mechanism. Mol Neurobiol. https://doi.org/10.1007/s12035-015-9376-y
Acknowledgements
AJ was supported by the National Science Center (grant Maestro no 2018/30/A/NZ3/00495). AS was supported by the Ministry of Science and Higher Education (0458/E-338/STYP/13/2018), National Science Center (grant Sonata no 2019/35/D/NZ3/04406) and Research Grant of DKMS Foundation. KS was supported by the Ministry of Science and Higher Education (0459/E‐338/STYP/13/2018), National Science Center (grant Harmonia no 2018/30/M/NZ5/00869) and Foundation for Polish Science (grant Homing no POIR.04.04.00-00-5F16/18-00). Schemes were created using images from Servier Medical Art https://smart.servier.com/ with modifications. We would like to apologize to any authors whose work we inadvertently left out.
Author information
Authors and Affiliations
Contributions
Conceptualization, review and editing: AJ and AS; literature search and figure preparation: AS and MM, original draft preparation: AS, KS and MM. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
AS, KS and AJ are the coinventors of the patents US10010557 and US10328085: Cobalt porphyrins for the treatment of blood-related disorders and EP 3139917: Cobalt protoporphyrin IX for the treatment of blood-related disorders granted to the Jagiellonian University. MM declares no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Szade, A., Szade, K., Mahdi, M. et al. The role of heme oxygenase-1 in hematopoietic system and its microenvironment. Cell. Mol. Life Sci. 78, 4639–4651 (2021). https://doi.org/10.1007/s00018-021-03803-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-021-03803-z