Abstract
Objective
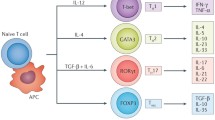
Myocardial infarction (MI) caused by ischemic cardiomyocyte necrosis induces inflammatory responses that strongly affect ventricular remodeling. Tolerogenic dendritic cells (tDCs) can suppress this effect on inflammatory responses. However, the precise role of atorvastatin-induced tDCs in ventricular remodeling after MI remains unclear.
Methods
To explore the effect of necrotic cardiomyocytes (SNC) and/or atorvastatin on DC function, the expression of CD40, CD80, CD86, and MHC-II was determined using flow cytometry. The protein levels of TLR-4/NF-κB-related molecules were evaluated using western blotting. The infarct area after MI was determined via 2,3,5-triphenyltetrazolium chloride staining. The TUNEL assay was employed to evaluate the apoptosis of cardiomyocytes in heart sections. Masson’s trichrome method was used to determine the extent of fibrosis.
Results
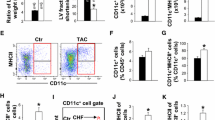
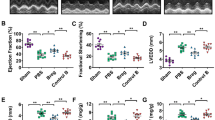
Compared to the DCs co-cultured with PBS (control), cells co-cultured with Supernatant-IM or Supernatant-NH produced higher levels of inflammatory cytokines, including TNF-α, IL-1, IL-6, IL-12P40, and IL-8. This cytokine production was impaired by atorvastatin treatment. SNC treatment induced DC maturation and enhanced inflammatory cytokine secretion and oxidative stress through TLR-4/NF-κB pathway activation. Compared to that in the PBS-treated group, the left ventricular ejection fraction was significantly improved after tDC treatment. Additionally, compared to that in the PBS-treated group, tDC treatment reduced the left ventricular end-diastolic and end-systolic diameters in mice. Furthermore, treatment with tDCs improved the left ventricular systolic function, attenuated inflammatory cell infiltration, and reduced cardiomyocyte apoptosis, myocardial fibrosis, and infarct size compared to those in the control group.
Conclusions
Adoptive transfer of atorvastatin-induced tDCs alleviated post-infarction cardiomyocyte apoptosis and myocardial fibrosis in association with decreased inflammatory cell infiltration and inhibited oxidative stress, likely by suppressing TLR-4/NF-κB activation after myocardial infarction.








Similar content being viewed by others
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
References
Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, et al. Heart disease and stroke statistics-2020 update: a report from the American Heart Association. Circulation. 2020;141:e139–596.
Zaidi Y, Aguilar EG, Troncoso M, Ilatovskaya DV, DeLeon-Pennell KY. Immune regulation of cardiac fibrosis post myocardial infarction. Cell Signal. 2021;77: 109837.
Liu J, Cao X. Cellular and molecular regulation of innate inflammatory responses. Cell Mol Immunol. 2016;13:711–21.
Timmers L, Sluijter JP, van Keulen JK, Hoefer IE, Nederhoff MG, Goumans MJ, et al. Toll-like receptor 4 mediates maladaptive left ventricular remodeling and impairs cardiac function after myocardial infarction. Circ Res. 2008;102:257–64.
Yano T, Miura T, Whittaker P, Miki T, Sakamoto J, Nakamura Y, et al. Macrophage colony-stimulating factor treatment after myocardial infarction attenuates left ventricular dysfunction by accelerating infarct repair. J Am Coll Cardiol. 2006;47:626–34.
Dieterlen MT, John K, Reichenspurner H, Mohr FW, Barten MJ. Dendritic cells and their role in cardiovascular diseases: a view on human studies. J Immunol Res. 2016;2016:5946807.
Ge J, Jia Q, Liang C, Luo Y, Huang D, Sun A, et al. Advanced glycosylation end products might promote atherosclerosis through inducing the immune maturation of dendritic cells. Arterioscler Thromb Vasc Biol. 2005;25:2157–63.
Huang D, Lu H, Liu H, Yao K, Sun A, Zou Y, et al. Losartan attenuates human monocyte-derived dendritic cell immune maturation via downregulation of lectin-like oxidized low-density lipoprotein receptor-1. J Cardiovasc Pharmacol. 2012;60:133–9.
Wu C, Gong Y, Yuan J, Zhang W, Zhao G, Li H, et al. microRNA-181a represses ox-LDL-stimulated inflammatory response in dendritic cell by targeting c-Fos. J Lipid Res. 2012;53:2355–63.
Zhu H, Jin X, Zhao J, Dong Z, Ma X, Xu F, et al. Probucol protects against atherosclerosis through lipid-lowering and suppressing immune maturation of CD11c+ dendritic cells in STZ-induced diabetic LDLR-/- mice. J Cardiovasc Pharmacol. 2015;65:620–7.
Zhu J, Yao K, Guo J, Shi H, Ma L, Wang Q, et al. miR-181a and miR-150 regulate dendritic cell immune inflammatory responses and cardiomyocyte apoptosis via targeting JAK1-STAT1/c-Fos pathway. J Cell Mol Med. 2017;21:2884–95.
Liu H, Gao W, Yuan J, Wu C, Yao K, Zhang L, et al. Exosomes derived from dendritic cells improve cardiac function via activation of CD4(+) T lymphocytes after myocardial infarction. J Mol Cell Cardiol. 2016;91:123–33.
Ma Y, Chen Z, Zou Y, Ge J. Atorvastatin represses the angiotensin 2-induced oxidative stress and inflammatory response in dendritic cells via the PI3K/Akt/Nrf 2 pathway. Oxid Med Cell Longev. 2014;2014: 148798.
Zhang Y, Wen W, Liu H. The role of immune cells in cardiac remodeling after myocardial infarction. J Cardiovasc Pharmacol. 2020;76:407–13.
Liu T, Liu J, Lin Y, Que B, Chang C, Zhang J, et al. IL-37 inhibits the maturation of dendritic cells through the IL-1R8-TLR4-NF-κB pathway. Biochim Biophys Acta Mol Cell Biol Lipids. 2019;1864:1338–49.
Ma Y, Ma L, Ma J, Wu R, Zou Y, Ge J. Hyperlipidemia inhibits the protective effect of lisinopril after myocardial infarction via activation of dendritic cells. J Cell Mol Med. 2020;24:4082–91.
Maekawa Y, Mizue N, Chan A, Shi Y, Liu Y, Dawood S, et al. Survival and cardiac remodeling after myocardial infarction are critically dependent on the host innate immune interleukin-1 receptor-associated kinase-4 signaling: a regulator of bone marrow-derived dendritic cells. Circulation. 2009;120:1401–14.
Gao E, Lei YH, Shang X, Huang ZM, Zuo L, Boucher M, et al. A novel and efficient model of coronary artery ligation and myocardial infarction in the mouse. Circ Res. 2010;107:1445–53.
Worbs T, Hammerschmidt SI, Förster R. Dendritic cell migration in health and disease. Nat Rev Immunol. 2017;17:30–48.
Anzai A, Anzai T, Nagai S, Maekawa Y, Naito K, Kaneko H, et al. Regulatory role of dendritic cells in postinfarction healing and left ventricular remodeling. Circulation. 2012;125:1234–45.
Zhu R, Sun H, Yu K, Zhong Y, Shi H, Wei Y, et al. Interleukin-37 and dendritic cells treated with interleukin-37 plus troponin I ameliorate cardiac remodeling after myocardial infarction. J Am Heart Assoc. 2016. https://doi.org/10.1161/JAHA.116.004406.
Choo EH, Lee JH, Park EH, Park HE, Jung NC, Kim TH, et al. Infarcted myocardium-primed dendritic cells improve remodeling and cardiac function after myocardial infarction by modulating the regulatory T cell and macrophage polarization. Circulation. 2017;135:1444–57.
Watts C, West MA, Zaru R. TLR signalling regulated antigen presentation in dendritic cells. Curr Opin Immunol. 2010;22:124–30.
Arnold-Schrauf C, Berod L, Sparwasser T. Dendritic cell specific targeting of MyD88 signalling pathways in vivo. Eur J Immunol. 2015;45:32–9.
Tavener SA, Long EM, Robbins SM, McRae KM, Van Remmen H, Kubes P. Immune cell Toll-like receptor 4 is required for cardiac myocyte impairment during endotoxemia. Circ Res. 2004;95:700–7.
Oesterle A, Laufs U, Liao JK. Pleiotropic effects of statins on the cardiovascular system. Circ Res. 2017;120:229–43.
Ma X, Liu S, Li T, Yuan H. Intensive statin treatment ameliorate the Th17/Treg functional imbalance in patients with non-ST elevation acute coronary syndrome underwent percutaneous coronary intervention. Clin Cardiol. 2020;43:379–85.
Tang TT, Yuan J, Zhu ZF, Zhang WC, Xiao H, Xia N, et al. Regulatory T cells ameliorate cardiac remodeling after myocardial infarction. Basic Res Cardiol. 2012;107:232.
Rangasamy T, Williams MA, Bauer S, Trush MA, Emo J, Georas SN, et al. Nuclear erythroid 2 p45-related factor 2 inhibits the maturation of murine dendritic cells by ragweed extract. Am J Respir Cell Mol Biol. 2010;43:276–85.
Qin T, Yin Y, Yu Q, Yang Q. Bursopentin (BP5) protects dendritic cells from lipopolysaccharide-induced oxidative stress for immunosuppression. PLoS ONE. 2015;10: e0117477.
Pazmandi K, Agod Z, Kumar BV, Szabo A, Fekete T, Sogor V, et al. Oxidative modification enhances the immunostimulatory effects of extracellular mitochondrial DNA on plasmacytoid dendritic cells. Free Radic Biol Med. 2014;77:281–90.
Marcusa DP, Giugliano RP, Park JG, de Lemos JA, Cannon CP, Sabatine MS. Association of baseline low-density lipoprotein cholesterol and percentage low-density lipoprotein cholesterol reduction with statins, ezetimibe, and PCSK9 inhibition. JAMA Cardiol. 2020;6:1–5.
Sabatine MS, Wiviott SD, Im K, Murphy SA, Giugliano RP. Efficacy and safety of further lowering of low-density lipoprotein cholesterol in patients starting with very low levels: a meta-analysis. JAMA Cardiol. 2018;3:823–8.
Shamshiev AT, Ampenberger F, Ernst B, Rohrer L, Marsland BJ, Kopf M. Dyslipidemia inhibits Toll-like receptor-induced activation of CD8alpha-negative dendritic cells and protective Th1 type immunity. J Exp Med. 2007;204:441–52.
Packard RR, Maganto-García E, Gotsman I, Tabas I, Libby P, Lichtman AH. CD11c(+) dendritic cells maintain antigen processing, presentation capabilities, and CD4(+) T-cell priming efficacy under hypercholesterolemic conditions associated with atherosclerosis. Circ Res. 2008;103:965–73.
Takenaka MC, Quintana FJ. Tolerogenic dendritic cells. Semin Immunopathol. 2017;39:113–20.
Cao X. Self-regulation and cross-regulation of pattern-recognition receptor signalling in health and disease. Nat Rev Immunol. 2016;16:35–50.
Yan X, Anzai A, Katsumata Y, Matsuhashi T, Ito K, Endo J, et al. Temporal dynamics of cardiac immune cell accumulation following acute myocardial infarction. J Mol Cell Cardiol. 2013;62:24–35.
Zhu J, Chen H, Ma Y, Liu H, Chen Z. Tolerogenic dendritic cells induced by atorvastatin via inhibition of the TLR-4/NF-κB pathway improve cardiac remodeling after myocardial infarction. ResearchGate. 2020. https://doi.org/10.21203/rs.3.rs-118076/v1.
Acknowledgements
This study was supported by the Natural Science Foundation of Jiangxi Province (Grant Nos. 20192ACBL21040 and 20204BCJ23017), National Natural Science Foundation of China (Grant Nos. 81800324, 81960061), and Qingdao Outstanding Health Professional Development Fund. A preprint has previously been published [39].
Author information
Authors and Affiliations
Contributions
QW, ZC, and JG performed the experiments and wrote the manuscript. XP and ZZ supervised the study and revised the manuscript. HL, YM and HC analyzed the data. JZ designed the experiment. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Consent for publication
All authors have approved the manuscript and have agreed to submit to your journal. This manuscript has not been published or presented elsewhere, in part or in its entirety, and is not under consideration by another journal.
Ethics approval and consent to participate
The study design was approved by the ethics committee of The First Affiliated Hospital of Nanchang University, Nanchang, Jiangxi Province. All procedures performed in studies involving animals were in accordance with the ethical standards of the Laboratory Animal Management and Experimental Ethics Committee of The First Affiliated Hospital of Nanchang University, Nanchang, Jiangxi Province. All methods were performed in accordance with relevant guidelines and regulations.
Additional information
Responsible Editor: John Di Battista.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, Q., Chen, Z., Guo, J. et al. Atorvastatin-induced tolerogenic dendritic cells improve cardiac remodeling by suppressing TLR-4/NF-κB activation after myocardial infarction. Inflamm. Res. 72, 13–25 (2023). https://doi.org/10.1007/s00011-022-01654-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-022-01654-3