Abstract
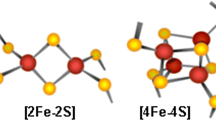
Iron sulfur proteins (Fe-S) are ancient structures present in archaebacteria and are involved in central metabolic functions in these ancient organisms (Iwasaki, Archaea 2010:1, 2010). From an evolutionary perspective, Fe-S clusters originated early on during protein evolution; with the aid of an inducible and robust antioxidant defense system, organisms have evolved to tackle oxygen toxicity and metabolism. Since Fe-S cluster proteins are known to be responsible for a variety of physiological functions in mammals, destabilization of the clusters by oxidative and nitrosative stress can lead to consequences such as TCA cycle downregulation, mitochondrial dysfunction and also oxidative stress-induced carcinogenesis. Indeed, mutations of some Fe-S proteins have been correlated with cancer. Particularly, cancer pathophysiology has been linked to oxidative stress. Also, defective Fe-S cluster assembly due to genetic abnormalities is the underlying cause for several other diseases, which are discussed in detail in Roland Lill and Tracey Rouault’s works (Sheftel et al. Trends Endocrinol Metab 21 (5):302–314, 2010; Rouault and Tong, Trends Genet 24 (8):398–407, 2008; Ye and Rouault, Biochemistry 49 (24):4945–4956, 2010; Lill and Mühlenhoff, Annu Rev Biochem 77:669–700, 2008). Lack of mature Fe-S clusters in respiratory complexes leads to low energy (fatigue) and metabolic dysfunction because lipoic acid synthesis and many metabolic pathways are regulated by Fe-S proteins. Also, Fe-S cluster depletion in nuclear Fe-S proteins which are involved in DNA synthesis, maintenance and repair can potentially spark carcinogenesis. Moreover, since Fe-S cluster proteins are powerful ‘sensors’ of oxidative stress, they are important signalling agents which can alert cells to oxidative stress damage, and hence, were termed as “sentinels” (Py et al. Curr Opin Microbiol 14 (2):218–223, 2011). Reaction of Fe-S clusters with reactive oxygen and nitrogen species is an inevitable consequence of oxygen-dependent respiration; hence, cells possess machinery to synthesize and repair damaged Fe-S clusters, to restore protein functionality and cellular viability. When the oxidative damage overwhelms the antioxidant defense system and impairs Fe-S repair/reconstitution, there can be dire consequences, including cancer.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
Abbreviations
- ATP:
-
Adenosine triphosphate
- BOLA1:
-
BolA Family Member 1
- CDGSH:
-
CDGSH iron sulfur domain 1 (MitoNEET)
- CIA:
-
Cytosolic iron-sulfur protein assembly
- DCA:
-
Dichloroacetate
- DNIC:
-
Dinitrosyl-Iron Complex
- DPYD:
-
Dihydropyrimidine Dehydrogenase
- ELP3:
-
Elongator complex protein 3
- EPR:
-
Electron paramagnetic resonance
- ETC:
-
Electron transport chain
- ETFDH:
-
Electron Transfer Flavoprotein Dehydrogenase
- FADH:
-
Flavin adenine dinucleotide, reduced
- FANCJ:
-
Fanconi anemia group J
- FMN:
-
Flavin mononucleotide
- FXN:
-
Frataxin
- GLRX5:
-
Glutaredoxin 5
- GPAT:
-
Glycerol-3-phosphate acyltransferase
- IRE-BP:
-
Iron-responsive element-binding protein
- IRP-1:
-
Iron regulatory protein
- ISCU:
-
Iron-Sulfur Cluster Assembly Enzyme
- MOCS1:
-
Molybdenum Cofactor Synthesis 1
- MUTYH:
-
mutY DNA glycosylase
- NADH:
-
Nicotinamide adenine dinucleotide
- NAF-1:
-
Nuclear Assembly Factor 1
- NDUFS:
-
NADH:Ubiquinone Oxidoreductase Core Subunit S
- NFS1:
-
Cysteine desulfurase
- NMR:
-
Nuclear Magnetic Resonance
- NTHL1:
-
Nth Like DNA Glycosylase 1
- PEITC:
-
β-Phenethyl isothiocyanate
- RNS:
-
Reactive Nitrogen Species
- ROS/RNS:
-
Reactive Oxygen species
- SDHB:
-
Succinate dehydrogenase [ubiquinone] iron-sulfur subunit, mitochondrial
- TCA:
-
Tricarboxylic acid cycle
- VDAC:
-
Voltage-dependent anion channels
- XDH:
-
Xanthine dehydrogenase
References
Beinert H (2000) Iron-sulfur proteins: ancient structures, still full of surprises. J Biol Inorg Chem 5(1):0002–0015
Beinert H, Holm RH, Münck E (1997) Iron-sulfur clusters: nature’s modular, multipurpose structures. Science 277(5326):653–659
Buss JL, Greene BT, Turner J, Torti FM, Torti SV (2004) Iron chelators in cancer chemotherapy. Curr Top Med Chem 4(15):1623–1635
Chen G, Chen Z, Hu Y, Huang P (2011) Inhibition of mitochondrial respiration and rapid depletion of mitochondrial glutathione by β-phenethyl isothiocyanate: mechanisms for anti-leukemia activity. Antioxid Redox Signal 15(12):2911–2921
de Choudens SO, Barras F (2017) Genetic, biochemical, and biophysical methods for studying Fe-S proteins and their assembly. In: Methods in enzymology, vol 595. Elsevier, pp 1–32. ISSN 0076-6879, ISBN 9780128119440, https://doi.org/10.1016/bs.mie.2017.07.015.
Ciccarone F, Di Leo L, Lazzarino G, Maulucci G, Di Giacinto F, Tavazzi B, Ciriolo MR (2020) Aconitase 2 inhibits the proliferation of MCF-7 cells promoting mitochondrial oxidative metabolism and ROS/FoxO1-mediated autophagic response. Br J Cancer 122(2):182–193
Crack JC, Green J, Cheesman MR, Le Brun NE, Thomson AJ (2007) Superoxide-mediated amplification of the oxygen-induced switch from [4Fe-4S] to [2Fe-2S] clusters in the transcriptional regulator FNR. Proc Natl Acad Sci 104(7):2092–2097
Davalli P, Mitic T, Caporali A, Lauriola A, D’Arca D (2016) ROS, cell senescence, and novel molecular mechanisms in aging and age-related diseases. Oxidative Med Cell Longev 2016:1
Droge W (2002) Free radicals in the physiological control of cell function. Physiol Rev 82(1):47–95
Favaro E, Ramachandran A, McCormick R, Gee H, Blancher C, Crosby M, Devlin C, Blick C, Buffa F, Li J-L (2010) MicroRNA-210 regulates mitochondrial free radical response to hypoxia and Krebs cycle in cancer cells by targeting iron sulfur cluster protein ISCU. PLoS One 5(4):e10345
Flint DH, Allen RM (1996) Iron− sulfur proteins with nonredox functions. Chem Rev 96(7):2315–2334
Florence T (1995) The role of free radicals in disease. Aust N Z J Ophthalmol 23(1):3–7
Fontecave M (2006) Iron-sulfur clusters: ever-expanding roles. Nat Chem Biol 2(4):171–174
Furihata T, Takada S, Maekawa S, Mizushima W, Watanabe M, Takahashi H, Fukushima A, Tsuda M, Matsumoto J, Kakutani N, Yokota T, Matsushima S, Otsuka Y, Matsumoto M, Nakayama KI, Nio-Kobayashi J, Iwanaga T, Sabe H, Hatakeyama S, Tsutsui H, Kinugawa S (2018) mitoNEET regulates mitochondrial iron homeostasis interacting with transferrin receptor. bioRxiv:330084. https://doi.org/10.1101/330084
Geldenhuys WJ, Long TE, Saralkar P, Iwasaki T, Nuñez RA, Nair RR, Konkle ME, Menze MA, Pinti MV, Hollander JM (2019) Crystal structure of the mitochondrial protein mitoNEET bound to a benze-sulfonide ligand. Commun Chem 2(1):1–9
Gideon DA, Nirusimhan V, Manoj KM (2020) Are plastocyanin and ferredoxin specific electron carriers or generic redox capacitors? Classical and murburn perspectives on two photosynthetic proteins. J Biomol Struct Dyn:1–15, https://doi.org/10.1080/07391102.2020.1835715
Guccini I, Serio D, Condo I, Rufini A, Tomassini B, Mangiola A, Maira G, Anile C, Fina D, Pallone F (2011) Frataxin participates to the hypoxia-induced response in tumors. Cell Death Dis 2(2):e123–e123
Halliwell B (2015) Free Radicals and Other Reactive Species in Disease. In eLS, John Wiley & Sons, Ltd (Ed.). https://doi.org/10.1002/9780470015902.a0002269.pub3
Huang M-E, Facca C, Fatmi Z, Baïlle D, Bénakli S, Vernis L (2016) DNA replication inhibitor hydroxyurea alters Fe-S centers by producing reactive oxygen species in vivo. Sci Rep 6:29361
Hwang PM, Bunz F, Yu J, Rago C, Chan TA, Murphy MP, Kelso GF, Smith RA, Kinzler KW, Vogelstein B (2001) Ferredoxin reductase affects p53-dependent, 5-fluorouracil–induced apoptosis in colorectal cancer cells. Nat Med 7(10):1111–1117
Iwasaki T (2010) Iron-sulfur world in aerobic and hyperthermoacidophilic archaea Sulfolobus. Archaea 2010:1
Johnson DC, Dean DR, Smith AD, Johnson MK (2005) Structure, function, and formation of biological iron-sulfur clusters. Annu Rev Biochem 74:247–281
Kohanski MA, Dwyer DJ, Hayete B, Lawrence CA, Collins JJ (2007) A common mechanism of cellular death induced by bactericidal antibiotics. Cell 130(5):797–810
Lill R, Freibert S-A (2020) Mechanisms of mitochondrial iron-sulfur protein biogenesis. Annu Rev Biochem 89:471
Lill R, Mühlenhoff U (2008) Maturation of iron-sulfur proteins in eukaryotes: mechanisms, connected processes, and diseases. Annu Rev Biochem 77:669–700
Lipper CH, Stofleth JT, Bai F, Sohn Y-S, Roy S, Mittler R, Nechushtai R, Onuchic JN, Jennings PA (2019) Redox-dependent gating of VDAC by mitoNEET. Proc Natl Acad Sci 116(40):19924–19929
Mahadevan A, Fernando S (2018) Inorganic iron-sulfur clusters enhance electron transport when used for wiring the NAD-glucose dehydrogenase based redox system. Microchim Acta 185(7):337
Manoj KM, Gideon DA (2020) Roles of cytochromes c and b5 in mitochondria and microsomes: classical and murburn perspectives
Manoj KM, Soman V, Jacob VD, Parashar A, Gideon DA, Kumar M, Manekkathodi A, Ramasamy S, Pakshirajan K, Bazhin NM (2019) Chemiosmotic and murburn explanations for aerobic respiration: predictive capabilities, structure-function correlations and chemico-physical logic. Arch Biochem Biophys 676:108128
Manoj KM, Gideon DA, Parashar A (2020) What is the role of lipid membrane-embedded quinones in mitochondria and chloroplasts? Chemiosmotic Q-cycle versus murburn reaction perspective. Cell Biochem Biophy 79:3–10. https://doi.org/10.1007/s12013-020-00945-y
Meyer J (2008) Iron–sulfur protein folds, iron–sulfur chemistry, and evolution. JBIC J Biol Inorg Chem 13(2):157–170
Murphy MP (2009) How mitochondria produce reactive oxygen species. Biochem J 417(1):1–13
Noodleman L, Peng C, Case D, Mouesca J-M (1995) Orbital interactions, electron delocalization and spin coupling in iron-sulfur clusters. Coord Chem Rev 144:199–244
Orme-Johnson W (1973) Iron-sulfur proteins: structure and function. Annu Rev Biochem 42(1):159–204
Paul VD, Lill R (2015) Biogenesis of cytosolic and nuclear iron–sulfur proteins and their role in genome stability. Biochim Biophy Acta (BBA)-Mol Cell Res 1853(6):1528–1539
Py B, Barras F (2010) Building Fe–S proteins: bacterial strategies. Nat Rev Microbiol 8(6):436–446
Py B, Moreau PL, Barras F (2011) Fe–S clusters, fragile sentinels of the cell. Curr Opin Microbiol 14(2):218–223
Roberts CK, Barnard RJ, Sindhu RK, Jurczak M, Ehdaie A, Vaziri ND (2006) Oxidative stress and dysregulation of NAD (P) H oxidase and antioxidant enzymes in diet-induced metabolic syndrome. Metabolism 55(7):928–934
Rouault TA (2019) The indispensable role of mammalian iron sulfur proteins in function and regulation of multiple diverse metabolic pathways. Biometals 32(3):343–353
Rouault TA, Tong WH (2008) Iron–sulfur cluster biogenesis and human disease. Trends Genet 24(8):398–407
Sahni S, Hickok JR, Thomas DD (2018) Nitric oxide reduces oxidative stress in cancer cells by forming dinitrosyliron complexes. Nitric Oxide 76:37–44
Sands RH, Beinert H (1960) Studies on mitochondria and submitochondrial particles by paramagnetic resonance (EPR) spectroscopy. Biochemical and Biophysical Research Communications 3(1):47–52. ISSN 0006-291X, https://doi.org/10.1016/0006-291X(60)90101-7.
Sheftel A, Stehling O, Lill R (2010) Iron–sulfur proteins in health and disease. Trends Endocrinol Metab 21(5):302–314
Sohn Y-S, Tamir S, Song L, Michaeli D, Matouk I, Conlan AR, Harir Y, Holt SH, Shulaev V, Paddock ML (2013) NAF-1 and mitoNEET are central to human breast cancer proliferation by maintaining mitochondrial homeostasis and promoting tumor growth. Proc Natl Acad Sci 110(36):14676–14681
Stehling O, Elsässer H-P, Brückel B, Mühlenhoff U, Lill R (2004) Iron–sulfur protein maturation in human cells: evidence for a function of frataxin. Hum Mol Genet 13(23):3007–3015
Toyokuni S (2009) Role of iron in carcinogenesis: cancer as a ferrotoxic disease. Cancer Sci 100(1):9–16
Wang P, Mai C, Wei Y-l, Zhao J-j, Hu Y-m, Zeng Z-l, Yang J, Lu W-h, Xu R-h, Huang P (2013) Decreased expression of the mitochondrial metabolic enzyme aconitase (ACO2) is associated with poor prognosis in gastric cancer. Med Oncol 30(2):552
Wilson DF, Erecinska M, Dutton PL, Tsudzuki T (1970) The oxidation-reduction potentials of the iron-sulfur proteins in mitochondria. Biochem Biophys Res Commun 41(5):1273–1278
Ye H, Rouault TA (2010) Human iron− sulfur cluster assembly, cellular iron homeostasis, and disease. Biochemistry 49(24):4945–4956
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 Springer Nature Singapore Pte Ltd.
About this entry
Cite this entry
James, J., Gideon, D.A.M., Roy, D., Mandal, A. (2022). Iron Sulfur Clusters and ROS in Cancer. In: Chakraborti, S., Ray, B.K., Roychoudhury, S. (eds) Handbook of Oxidative Stress in Cancer: Mechanistic Aspects. Springer, Singapore. https://doi.org/10.1007/978-981-15-9411-3_24
Download citation
DOI: https://doi.org/10.1007/978-981-15-9411-3_24
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-9410-6
Online ISBN: 978-981-15-9411-3
eBook Packages: Biomedical and Life SciencesReference Module Biomedical and Life Sciences