Abstract
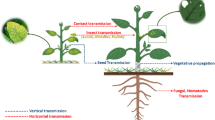
Plant virus diseases cause enormous loss which is estimated to be US$60 billion in crop yields worldwide each year. Seed is a propagating material in most of the crop plants, whereas most of the horticultural crops are vegetatively propagated. Horticultural crops like banana, bamboo, citrus, and grapes; commercial crops like sugarcane, black pepper, cardamom, orchids, and bulbous ornamentals; and tuber crops like potato, cassava, yam, etc., have been known to be infected by a range of viruses that belong to different genera and families. In these crops, the primary mode of transmission of viruses is through the use of infected plant propagule like corms, tubers, cutting, grafts, etc. An effective virus management strategy requires an accurate, rapid, and sensitive diagnosis for which understanding the disease cycle of etiological agents and its molecular nature, genome sequence and structure, coat protein information and sequences, etc. should be known in advance to design a detection strategy. Enzyme-linked immunosorbent assay (ELISA) or polymerase chain reaction (PCR) has been widely used for detection of plant viruses. These methods are time-consuming and laborious and require special skills such as in prior information on taxonomy to detect the pathogen responsible for the disease. On-site or point-of-care methods of detection are not new but limited to clinical use for human diseases. But recently this lateral flow devices (LFDs) are being made available for a number of viruses infecting plants. However, the widespread usage of this technology is delayed probably due to its limitation on robustness and lack of high-throughput nature. In this chapter, we have reviewed the recent developments on the early diagnosis using cutting-edge technologies like on-the-spot diagnostic tool lateral flow immunoassay (LFIA), loop-mediated isothermal amplification (LAMP), and multiplex technologies like microarray, microsphere immunoassay, etc. LAMP method is highly specific and requires less time to complete the indexing; however, this technique is yet to replace ELISA and PCR completely in agriculture possibly due to its specificity and viral variants that might escape from detection, and other possible reasons may be that the researchers and policy makers have not yet been convinced. Recent technologies such as rolling circle amplification which is dependent on a circular DNA genome and random hexamers do not require sequence data of the target, and similarly the next-generation sequencing also does not require a priori knowledge on the sequence of the causal agents. Principles and application of these cutting-edge technologies are reviewed in this chapter. The objective of the present chapter is not to cover all the details of diagnostics but to highlight the current status of various cutting-edge diagnostic techniques that can be applied for the biosecurity, by the quarantine departments, international exchange of germplasm, and on-site field detection by farmers, and use in the certification programs.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Abdullahi I, Rott M (2009) Microarray immunoassay for the detection of grapevine and tree fruit viruses. J Virol Methods 160:90–100
Abdullahi I, Koerbler M, Stachewicz H, Winter S (2005) The 18S rDNA sequence of Synchytrium endobioticum and its utility in micro arrays for the simultaneous detection of fungal and viral pathogens of potato. Appl Microbiol Biotechnol 68:368–375
Adams IP, Glover RH, Monger WA, Mumford R, Jackeviciene E, Navalin-skiene M, Samuitiene M, Boonham N (2009) Next-generation sequencing and metagenomic analysis: a universal diagnostic tool in plant virology. Mol Plant Pathol 10(4):537–545
Agindotan B, Perry KL (2007) Macroarray detection of plant RNA viruses using randomly primed and amplified complementary DNAs fromiInfected plants. Phytopathology 97:119–127
Agindotan B, Perry KL (2008) Macroarray detection of eleven potato-infecting viruses and potato spindle tuber viroid. Plant Dis 92:730–740
Ahlawat YS, Pant RP, Lockhart BEL, Srivastava M, Chakraborty NK, Varma A (1996) Association of badnavirus with citrus mosaic disease in India. Plant Dis 80:590–592
Ahmadi S, Almasi MA, Fatehi F, Struik PC, Moradi A (2013) Visual Detection of Potato leafroll virus by One-step Reverse Transcription Loop-Mediated Isothermal Amplification of DNA with Hydroxynaphthol Blue Dye. J Phytopathol 161:120–124
Al Rwahnih M, Daubert S, Golino D, Rowhani A (2009) Deep sequencing analysis of RNAs from a grapevine showing Syrah decline symptoms reveals a multiple virus infection that includes a novel virus. Virology 387:395–401
Al Rwahnih M, Sudarshana MR, Uyemoto JK, Rowhani A (2012a) Complete genome sequence of a novel Vitivirus isolated from grapevine. In: Proceedings of the 17th Congress of ICVG, Davis, CA, USA, 7–14 October 2012
Al Rwahnih M, Dave A, Anderson M, Uyemoto JK, Sudarshana MR (2012b) Association of a circular DNA virus in grapevine affected by red blotch disease in California. In: Proceedings of the 17th Congress of ICVG, Davis, CA, USA, 7–14 October 2012
Alabi OJ, Zheng Y, Jagadeeswaran G, Sunkar R, Naidu RA (2012) High-throughput sequence analysis of small RNAs in grapevine (Vitis viniferaL.) affected by grapevine leafroll disease. Mol Plant Pathol 13(9):1060–1076
Algar WR, Krull UJ (2007) Quantum dots as donors in fluorescence resonance energy transfer for the bioanalysis of nucleic acids, proteins, and other biological molecules. Anal Bioanal Chem 39:1609–1618
Alivisatos AP, Gu W, Larabell C (2005) Quantum dots as cellular probes. Biomed Eng 7:55–76
Almasi MA, Dehabadi SH (2013) Colorimetric Immunocapture Reverse Transcription Loop-Mediated Isothermal Amplification Assay for Rapid Detection of the Potato virus Y. J Plant Pathol Microbiol 4:188. doi:10.4172/2157-7471.1000188
Almasi MA, Moradi A, Nasiri J, Karami S, Nasiri M (2012) Assessment of performance ability of three diagnostic methods for detection of Potato Leafroll virus (PLRV) using different visualizing systems. Applied Biochem Biotechnol 168:770–784
Almasi MA, Manesh ME, Jafary H, Dehabadi SMH (2013) Visual detection of Potato Leafroll Virus by loop-mediated isothermal amplification of DNA with the Gene Finder TM dye. J Virological Methods 192:51–54
Anthony Johnson AM, Dasgupta I, Sai Gopal DVR (2014) Development of loop-mediated isothermal amplification and SYBR green real-time PCR methods for the detection of Citrus yellow mosaic badnavirus in citrus species. J Virol Methods 203:9–14
Baptista PV, Koziol-Montewka M, Paluch-Oles J, Doria G, Franco R (2006) Gold-nanoparticle-probe-based assay for rapid and direct detection of Mycobacterium tuberculosis DNA in clinical samples. Clin Chem 52:1433–1434
Barba M, Czosnek H, Hadidi A (2014) Historical perspective, development and applications of next-generation sequencing in plant virology. Viruses 6(1):106–136
Bar-Joseph M, Gera A (2012) On a revolutionary method for diagnosis of latent infections of viruses and the importance of its adoption to prevent the spread of citrus and other fruit tree diseases (in Hebrew). Alon Hanotea 66:46–48
Bergervoet JH, Peters J, van Beckhoven JR, van den Bovenkamp GW, Jacobson JW et al (2008) Multiplex microsphere immuno-detection of potato virus Y, X and PLRV. J Virol Methods 149:63–68
Bhat AI, Siljo A, Deeshma KP (2013) Rapid detection of Piper yellow mottle virus and Cucumber mosaic virus infecting black pepper(Piper nigrum) by loop- mediated isothermal amplification(LAMP). J Virol Methods 193:190–196
Boonham N, Walsh K, Smith P, Madagan K, Graham I, Barker I (2003) Detection of potato viruses using microarray technology: towards a generic method for plant viral disease diagnosis. J Virol Methods 108:181–187
Boonham N, Kreuze J, Winter S, van der Vlugt R, Bergervoet J, Tomlinson J, Mumford R (2014) Methods in virus diagnostics: from ELISA to next generation sequencing. Virus Res 186:20–31
Bruchez M Jr, Moronne M, Gin P, Weiss S, Alivisatos AP (1998) Semiconductor nanocrystals as fluorescent biological labels. Science 281:2013–6
Bystricka D, Lenz O, Mraz I, Dedic P, Sip M (2003) DNA microarrays: parallel detection of potato viruses. Acta Virol 47:41–44
Bystricka D, Lenz O, Mraz I, Piherova L, Kmoch S, Sip M (2005) Oligonucleotide-based microarray: A new improvement in microarray detection of plant viruses. J Virol Methods 128:176–182
Byzova NA, Safenkova IV, Chirkov SN, Zherdev AV, Blintsov AN, Dzantiev BB, Atabekov IG (2009) Development of Immunochromatographic test systems for express detection of plant viruses. Appl Biochem Microbiol 45:204–209
Byzova NA, Safenkova IV, Chirkov SN, Avdienko VG, Guseva AN, Mitrofanova IV, Zherdev AV, Dzantiev BB, Atabekov JG (2010) Interaction of Plum Pox Virus with Specific Colloidal Gold Labeled Antibodies and Development of Immunochromatographic Assay of the Virus. Biokhimiya 75(11):1583–1595
Candresse T, Marais A, Faure C, Carriere S, Gentit P (2012) Use of 454 pyrosequencing for the fast and efficient characterization of known or novel viral agents in Prunus materials. In: Proceedings of the 22nd international conference on virus and other transmissible diseases of fruit crops, Rome, Italy, 3–8 June 2012; Abstract No. 47
Candresse T, Marais A, Faure C, Gentit P (2013) Association of Little cherry virus 1 (LChV1) with the Shirofugen stunt disease and characterization of the genome of a divergent LChV1 isolate. Phytopathology 103:293–308
Charlermroj R, Himananto O, Seepiban C, Kumpoosiri M, Warin N et al (2013) Multiplex Detection of Plant Pathogens Using a Microsphere Immunoassay Technology. PLoS One 8(4), e62344. doi:10.1371/journal.pone.0062344
Chiumenti M, Roberto R, Bottalico G, Campanale A, de Stradis A, Minafra A, Boscia D, Savino V, Martelli GP (2012) Virus sanitation and deep sequence analysis of fig. In: Proceedings of the 22nd international conference on virus and other transmissible diseases of fruit crops, Rome, Italy, 3–8 June 2012; Abstract No. 163
Coetzee B, Freeborough MJ, Maree HJ, Celton JM, Rees DJ, Burger JT (2010) Deep sequencing analysis of viruses infecting grapevines: Virome of a vineyard. Virology 400:157–163
Dale JL (1987) Banana bunchy top: an economically important tropical plant virus disease. Adv Virus Res 33:301–325
Danks C, Barker I (2000) On-site detection of plant pathogens using lateral-flow devices. OEPP/EPPO Bull 30:421–426
Dean FB, Nelson JR, Giesler TL, Lasken RS (2001) Rapid amplification of plasmid and phage DNA using Phi29 polymerase and multiply-primed rolling circle amplification. Genome Res 11:1095–1099
Deyong Z, Willingmann P, Heinze C, Adam G, Pfunder M, Frey B, Frey JE (2005) Differentiation of Cucumber mosaic virus isolates by hybridization to oligonucleotides in a microarray format. J Virol Methods 123:101–108
Dombrovsky A, Glanz E, Sapkota R, Lachman O, Bronstein M, Schnitzer T, Antignus Y (2011) Next-generation sequencing a rapid and reliable method to obtain sequence data of the genomes of un described plant viruses. In: Proceedings of the BARD-Sponsored workshop—microarrays and next-generation sequencing for detection and identification of plant viruses, Beltsville, MD, USA, 17–19 November 2011; Abstract No. 24
Drygin YF, Blintsov AN, Osipov AP, Grigorenko VG, Andreeva IP, Uskov AI, Varitsev YA, Anisimov BV, Novikov VK, Atabekov JG (2009) High-sensitivity express immunochromatographic method for detection of plant infection by tobacco mosaic virus. Biochem Moscow 74:986–993
Drygin YF, Blintsov AN, Osipov AP, Grigorenko VG, Andreeva IP, Osipov AP, Varitsev YA, Uskov AI, Kravchenko DV, Atabekov JG (2012) Highly sensitive field test lateral flow immunodiagnostics of PVX infection. Appl Microbiol Biotechnol 93:179–189
Dunbar SA, Vander Zee CA, Oliver KG, Karem KL, Jacobson JW (2003) Quantitative, multiplexed detection of bacterial pathogens: DNA and protein applications of the Luminex LabMAPTM system. J Microbiol Methods 53:245–252
Edgar R, McKinstry M, Hwang J, Oppenheim AB, Fekete RA, Giulian G, Merril C, Nagashima K, Adhya S (2006) High sensitivity bacterial detection using biotin tagged phage and quantum-dot nanocomplexes. Proceeding of National Academy of. Science U. Space Sci Rev 103:4841–4845
Engel EA, Escobar PF, Rojas LA, Rivera PA, Fiore N, Valenzuela PD (2010) A diagnostic oligonucleotide microarray for simultaneous detection of grapevine viruses. J Virol Methods 163(2):445–451
Foord AJ, White JR, Colling A, Heine HG (2013) Microsphere suspension array assays for detection and differentiation of Hendra and Nipah viruses. Bio Med Res Int 2 [Article ID 89295]
Frasco MF, Chaniotakis N (2009) Semiconductor quantum dots in chemical sensors and biosensors. Sensors 9:7266–7286
Fukuta S, Nimi Y, Oishi K, Yoshimura Y, Anai N, Hotta M, Fukaya M, Kato T, Oya T, Kambe M (2005) Development of reverse transcription loop-mediated isothermal amplification (RT-LAMP) method for detection of two viruses and chrysanthemum stunt viroid. Ann Rep Kansai Plant Protection 47:31–36
Giampetruzzi A, Roumi V, Roberto R, Malossini U, Yoshikawa N, la Notte P, Terlizzi F, Credi R, Saldarelli P (2012) A new grapevine virus discovered by deep sequencing of virus- and viroid-derived small RNAs in Cv Pinot gris. Virus Res 163:262–268
Goldman ER, Mattoussi H, Anderson GP, Medintz IL, Mauro JM (2005) Fluoroimmunoassays using antibodyconjugated quantum dots. Methods Mol Biol 303:19–34
Goto M, Honda E, Ogura A, Nomoto A, Hanaki KI (2009) Colorimetric detection of loop-mediated isothermal amplification reaction by using hydroxy naphthol blue. Bio Techniques 46:167–172
Grover V, Pierce ML, Hoyt P, Zhang F, Melcher U (2010) Oligonucleotide-based microarray for detection of plant viruses employing sequence-independent amplification of targets. J Virol Methods 163(1):57–67
Hadidi A, Barba M (2012) Next-generation sequencing: Historical perspective and current applications in plant virology. Petria 22:262–277
Haible D, Kober S, Jeske H (2006) Rolling circle amplification revolutionizes diagnosis and genomics of geminiviruses. J Virol Methods 135:9–16
Hany U, Adams IP, Glover R, Bhat AI, Boonham N (2014) The complete nucleotide sequence of Piper yellow mottle virus (PYMoV). Arch Virol 159:385–388. doi:10.1007/s00705-013-1824-2
Jain KK (2005) Nanotechnology in clinical laboratory diagnostics. Clin Chim Acta 358:37–54
Johne R, Müller H, Rector A, van Ranst M, Stevens H (2009) Rolling-circle amplification of viral DNA genomes using phi29 polymerase. Trends Microbiol 17:205–211
Kaneko H, Kawana T, Fukushima E, Suzutani T (2007) Tolerance of loop-mediated isothermal amplification to a culture medium and biological substances. J Biochem Biophys Methods 70:499–501
Kaweesi T, Kawuki R, Kyaligonza V, Baguma Y, Tusiime G, Ferguson EM (2014) Field evaluation of selected cassava genotypes for cassava brown streak disease based on symptom expression and virus load. Virol J 11:216
Kellar KL, Douglass JP (2003) Multiplexed microsphere-based flow cytometric immunoassays for human cytokines. J Immunol Methods 279:277–285
Kreuze JF, Perez A, Untiveros M, Quispe D, Fuentes S, Barker I, Simon R (2009) Complete viral genome sequence and discovery of novel viruses by deep sequencing of small RNAs: A generic method for diagnosis, discovery and sequencing of viruses. Virology 388:1–7
Kuan CP, Wu MT, Lu YL, Huang HC (2010) Rapid detection of squash leaf curl virus by loop-mediated isothermal amplification. J Virol Methods 169:61–65
Kusano N, Hirashima K, Narahara MKK, Imamura T, Nakahira TMK, Torii K (2007) Immunochromatographic assay for simple and rapid detection of Satsuma dwarf virus and related viruses using monoclonal antibodies. J Gen Plant Pathol 73:66–71
Lee GP, Min BE, Kim CS, Choi SH, Harn CH, Kim SU, Ryu KH (2003) Plant virus cDNA chip hybridization for detection and differentiation of four cucurbit-infecting Tobamoviruses. J Virol Methods 110(1):19–24
Lee MS, Yang MJ, Hseu YC, Lai GH, Chang WT, Hsu YH, Lin MK (2011) One-step reverse transcription loop-mediated isothermal amplification assay for rapid detection of Cymbidium mosaic virus. J Virol Methods 173:43–48
Li R, Ling K (2014) Development of reverse transcription loop-mediated isothermal amplification assay for rapid detection of an emerging potyvirus: Tomato necrotic stunt virus. J Virol Methods 200:35–40
Li JY, Wei QW, Liu Y, Zhang WN, Wu JY, Charimbu MK, Hu BS, Cheng ZB, Yu C, Tao XR (2013) One-step reverse transcription loop-isothermal amplification for the rapid detection of cucumber green mottle mosaic virus. J Virol Methods 193:583–588
Lin A, Nguyen L, Lee T, Clotilde LM, Kase JA, Son I et al (2011) Rapid Osero group identification of the ten most clinically relevant STECs by Luminex microbead-based suspension array. J Microbiol Methods 87:105–110
Ling KS, Li R, Bledsoe M (2013) Pepino mosaic virus genotype shift in North America and development of a loop-mediated isothermal amplification for rapid genotype identification. Virol J 10:117
Liu K, Chen H, Zhou Y, Li Z (2015) Establishment of RT-LAMP Assay for Detection of Citrus yellow vein clearing virus. Acta Horticult Sinica 42(5):997–1002
Loconsole G, Onelge N, Potere O, Giampetruzzi A, Bozan O, Satar S, de Stradis A, Savino V, Yokomi RK, Saponari M (2012a) Identification and characterization of Citrus Yellow vein clearing virus, a putative new member of the genus Mandarivirus. Phytopathology 102:1168–1175
Loconsole G, Saldarelli P, Doddapaneni H, Savino V, Martelli GP, Saponari M (2012b) Identification of a single-stranded DNA virus associated with citrus chlorotic dwarf disease, a new member of the family Geminiviridae. Virology 432:162–172
Maree HJ, Nel Y, Visser M, Coetzec B, Manicom B, Burger JT, Rees DJG (2012) The study of plant virus disease etiology using next-generation sequencing technologies. In: Proceedings of the 22nd international conference on virus and other transmissible diseases of fruit crops, Rome, Italy, 3–8 June 2012; Abstract No. 48
Martelli GP (2012) Grapevine virology highlights. In: Proceedings of the 17th Congress of ICVG, Davis, CA, USA, 7–14 October 2012
Martelli GP, Boudon-Padieu E (2006) Directory of infectious diseases of grapevines. International Centre for Advanced Mediterranean Agronomic Studies. Options Méditerranéennes Ser B Studies Res 55:59–75
Massart S, Olmos A, Jijakli H, Candresse T (2014) Current impact and future directions of high throughput sequencing in plant virus diagnostics. Virus Res 188:90–96
Mirkin CA, Letsinger RL, Mucic RC, Storhoff JJ (1996) A DNA-based method for rationally assembling nanoparticles into macroscopic materials. Nature 382:607–609
Monger WA, Alicai T, Ndunguru J, Kinyua ZM, Potts M, Reeder RH, Miano DW, Adams IP, Boonham N, Glover RH et al (2010) The complete genome sequence of the Tanzanian strain of Cassava brown streak virus and comparison with the Ugandan strain sequence. Arch Virol 2010(155):429–433
Muerhoff AS, Leary TP, Desai SM, Mushahwar IK (1997) Amplification and subtraction methods and their application to the discovery of novel human viruses. J Medical Virol 53:96–103
Mukasa SB, Rubaihayo SB, Valkonen JPT (2006) Interactions between a crinivirus, an ipomovirus and a potyvirus in coinfected sweet potato plants. Plant Pathol 55:458–467
Muthukumar V, Melcher U, Pierce M, Wiley GB, Roe BA, Palmer MW, Thapa V, Ali A, Ding T (2009) Non-cultivated plants of the Tallgrass Prairie Preserve of northeastern Oklahoma frequently contain virus-like sequences in particulate fractions. Virus Res 141(2):169–173
Nagamine K, Kuzuhara Y, Notomi T (2002) Isolation of single-stranded DNA from loop-mediated isothermal amplification products. Biochem Biophys Res Commun 290:1195–1198
Nath S, Kaittanis C, Tinkham A, Perez JM (2008) Dextran coated gold nanoparticles for the assessment of antimicrobial susceptibility. Annal Chem 80:1033–1038
Ng JCK (2013) A quantum dot-immunofluorescent labeling method to investigate the interactions between a crinivirus and its whitefly vector. Front Microbiol 4(77):1–9
Notomi T, Okayama H, Masubuchi H, Yonekawa T, Watanabe K, Amino N et al (2000) Loop-mediated isothermal amplification of DNA. Nucleic Acids Res 28, e63
Olmos A, Dasí MA, Candresse T, Cambra M (1996) Print-capture PCR: a simple and highly sensitive method for the detection of Plum pox virus (PPV) in plant tissues. Nucleic Acids Res 24(11):2192–2193
Osman F, Leutenegger C, Golino D, Rowhani A (2008) Comparison of low-density arrays, RT-PCR and real-time TaqMan RT-PCR in detection of grapevine viruses. J Virol Methods 149:292–299
Pantaleo V, Saldarelli P, Miozzi L, Giampetruzzi A, Gisel A, Moxon S, Dalmay T, Bisztray G, Burgyan J (2010) Deep sequencing analysis of viral short RNAs from an infected Pinot noir grapevine. Virology 408:49–56
Pasquini G, Barba M, Hadidi A, Faggioli F, Negri R, Sobol I, Tiberini A, Caglayan K, Mazyad H, Anfoka G, Ghanim M, Zeidan M, Czosnek H (2008) Oligonucleotide microarray-based detection and genotyping of Plum pox virus. J Virol Methods 147(1):118–126
Peng J, Zhang J, Xia Z, Li Y, Huang J, Fan Z (2012a) Rapid and sensitive detection of Banana bunchy top virus by loop-mediated isothermal amplification. J Virol Methods 185:254–258
Peng J, Fan Z, Huang J (2012b) Rapid detection of banana streak virus by loop-mediated isothermal amplification assay in South China. J Phytopathol 160:248–250
Peng J, Shi M, Xia Z, Huang J, Fan Z (2012c) Detection of cucumber mosaic virus isolates from banana by one step reverse transcription loop-mediated isothermal amplification. Arch Virol 157:2213–2217
Poojari S, Alabi OJ, Fofanov VY, Naidu RA (2013) A leafhopper-transmissible DNA virus with novel evolutionary lineage in the family Geminiviridae implicated in grapevine red leaf disease by next-Generation sequencing. PLoS One 8, e64194
Prabha K, Baranwal VK, Jain RK (2013) Applications of next generation high throughput sequencing technologies in characterization, discovery and molecular interaction of plant viruses. Indian J Virol 24(2):157–165
Price CP, Kricka LJ (2007) Improving healthcare accessibility through point –of – care technologies. Clin Chem 53:1665–1675
Przewodowska A, Zacharzewska B, Chołuj J, Treder K (2015) A One-step, real-time reverse transcription loop-mediated isothermal amplification assay to detect potato virus Y. Am J Potato Res 92:303–311
Quito-Avila DF, Jelkmann W, Tzanetakis I, Keller K, Martin RR (2011) Complete sequence and genetic characterization of Raspberry latent virus, a novel member of the family Reoviridae. Virus Res 155:397–405
Reagin MJ, Giesler TL, Merla AL, Resetar-Gerke JM, Kapolka KM, Mamone JA (2003) TempliPhi: A sequencing template preparation procedure that eliminates overnight cultures and DNA purification. J Biomol Tech 14:143–148
Rodrigues JCV (2000) The relationships among the pathogen, vector and plant in the citrus leprosis system. Spo Paulo University, Piracicaba, SP, Brazil, PhD Dissertation
Rosi NL, Mirkin CA (2005) Nanostructures in biodiagnostics. Chem Rev 105:1547–1562
Roy A, Choudhary N, Guillermo LM, Shao J, Govindarajulu A, Achor D, Wei G, Picton DD, Levy L, Nakhla MK et al (2013) A novel virus of the Genus Cilevirus causing symptoms similar to citrus leprosis. Phytopathology 103:488–500
Ruiz-Ruiz S, Navarro B, Gisel A, Pena L, Navarro L, Moreno P, di Serio F, Flores R (2011) Citrus tristeza virus infection induces the accumulation of viral small RNAs (21–24 nt) mapping preferentially at the terminal region 3′- of the genomic RNA and affects the host small RNA profile. Plant Mol Biol 75:607–619
Safarpour H, Safarnejad MR, Tabatabaei M, Mohsenifar A, Rad F, Basirat M, Shahryari F, Hasanzadeh F (2012) Development of a quantum dots FRET-based biosensor for efficient detection of Polymyxa betae. Can J Plant Pathol 34(4):507–515
Safenkova I, Zherdev A, Dzantiev B (2012) Factors influencing the detection limit of the lateral-flow sandwich immunoassay: a case study with potato virus X. Anal Bio anal Chem 403:1595–1605
Salomone A, Roggero P (2002) Host range, seed transmission and detection by ELISA and lateral flow of an Italian isolate of Pepino mosaic virus. J Plant Pathol 84:65–68
Salomone A, Bruzzone C, Minuto G, Minuto A, Roggero P (2002) A comparison of lateral flow and ELISA for the detection of Tomato mosaic virus in tomato. J Plant Pathol 84:193
Salomone A, Mongelli M, Roggero P, Boscia D (2004) Reliability of detection of Citrus tristeza virus an immunochromatographic flow assay in comparison with ELISA. J Plant Pathol 86:43–48
Sastry KS (2013) Plant virus and viroid diseases in the tropics. Volume-1: Introduction of plant viruses and sub-viral agents, classification, assessment of loss, transmission and diagnosis. Springer, New York p 361
Seif AA (1982) Effect of cassava mosaic virus on yield of cassava. Plant Dis 66:661–662
Shen W, Tuo D, Yana P, Li X, Zhoua P (2014) Detection of Papaya leaf distortion mosaic virus by reverse-transcription loop-mediated isothermal amplification. J Virol Methods 195:174–179
Siljo A, Bhat AI (2014) Reverse transcription loop-mediated isothermal amplification assay for rapid and sensitive detection of Banana bract mosaic virus in cardamom (Elettaria cardamomum). Eur J Plant Pathol 138:209–214
Sip M, Bystricka D, Kmoch S, Noskova L, Hartmannova H, Dedic P (2010) Detection of viral infections by an oligonucleotide microarray. J Virol Methods 165(1):64–70
Sugiyama S, Masuta C, Sekiguchi H, Uehara T, Shimura H, Maruta Y (2008) A simple, sensitive, specific detection of mixed infection of multiple plant viruses using macroarray and microtube hybridization. J Virol Methods 153:241–244
Thapa V, Melcher U, Wiley GB, Doust A, Palmer MW, Roewe K, Roe BA, Shen G, Roossinck MJ, Wang YM, Kamath N (2012) Detection of members of the Secoviridae in the Tall grass Prairie Preserve, Osage County, Oklahoma, USA. Virus Res 167(1):34–42
Thekke-Veetil T, Sabanadzovic S, Keller KE, Martin RR, Tzanetakis IE (2012) Genome organization and sequence diversity of a novel blackberry Ampelovirus. In: Proceedings of the 22nd international conference on virus and other transmissible diseases of fruit crops, Rome, Italy, 3–8 June 2012; Abstract No. 191
Thompson JR, Fuchs M, McLane H, Celebi-Toprak F, Fischer KF, Potter JL et al (2014) Profiling viral infections in grapevine using a randomly primed reverse transcription-polymerase chain reaction/macroarray multiplex platform. Phytopathology 104:211–219
Tiberini A, Tomassoli L, Barba M, Hadidi A (2010) Oligonucleotide microarray-based and identification of ten major tomato viruses. J Virol Methods 168:133–140
Tomlinson JA, Boonham N, Dickinson M (2010) Development and evaluation of a one-hour DNA extraction and loop-mediated isothermal amplification assay for rapid detection of phytoplasmas. Plant Pathol 59:465–471
Tomlinson JA, Ostoja-Starzewska S, Adams IP, Miano DW, Abidrabo P, Kinyua Z, Alicai T, Dickinson MJ, Peters D, Boonham N, Smith J (2013) Loop-mediated isothermal amplification for rapid detection of the causal agents of cassava brown streak disease. J Virol Methods 191:148–154
Van Brunschot SL, Bergervoet JHW, Pagendam DE, de Weerdt M, Geering ADW, Drenth A et al (2014) Development of a multiplexed bead-based suspension array for the detection and discrimination of pospiviroid plant pathogens. PLoS One 9(1), e84743
Vives MC, Velazquez K, Pina JA, Moreno P, Guerri J, Navarro L (2013) Identification of a new enamovirus associated with citrus vein enation disease by deep sequencing of small RNAs. Phytopathology 103:1077–1086
Von Lode P (2005) Point-of-care immune-testing: approaching the analytical performance of central laboratory methods. Clin Biochem 38:591–606
Walsha HA, Pietersen G (2013) Rapid detection of Grapevine leafroll-associated virus type 3 using a reverse transcription loop-mediated amplification method. J Virol Methods 194:308–316
Wang D, Coscoy L, Zylberberg M, Avila PC, Boushey HA, Ganem D, DeRisi JL (2002) Microarray-based detection and genotyping of viral pathogens. Proc Natl Acad Sci U S A 99(24):15687–15692
Wang YJ, Zhou Y, Li ZA et al (2013) A RT-LAMP assay for detection of citrus tristeza virus. J China Agric Sci 46(3):517–524
Wang ZY, Gu QS, Sun H, Li HL, Sun BJ, Liang XZ, Yuan Y, Liu RL, Shi Y (2014) One-step reverse transcription loop mediated isothermal amplification assay for sensitive and rapid detection of Cucurbit chlorotic yellows virus. J Virol Methods 195:63–66
Warren M, Krüger K, Schoeman AS (2005) Potato virus Y ad potato leaf roll virus. University of Pretoria, A South African Perspective
Wei T, Pearson MN, Blohm D, Nolte M, Armstrong K (2009) Development of a short oligonucleotide microarray for the detection and identification of multiple potyviruses. J Virol Methods 162:109–118
Wetzel T, Candresse T, Macquaire G, Ravelonandro M, Dunez J (1992) A highly sensitive immunocapture polymerase chain reaction method for plum pox potyvirus detection. J Virolological Methods 39(1–2):27–37
Wyant PS, Strohmeier S, Schäfer B, Krenz B, Assunҫão IP, Lima GSDA, Jeske H (2012) Circular DNA genomics (circomics) exemplified for geminiviruses in bean crops and weeds of northeastern Brazil. Virology 427(2):151–157
Yoon JY, Choi GS, Cho IS, Choi SK (2014) Development of rapid immune-gold strip kit for on-site diagnosis of tomato spotted wilt virus. Res Plant Disease 20(1):15–20
Yoshikawa N, Yamagishi N, Yaegashi H, Ito T (2012) Deep sequence analysis of viral small RNAs from a green crinkle-diseased apple tree. Petria 22:292–297
Zhang Y, Yin J, Li G, Li M, Huang X, Chen H, Zhao W, Zhu S (2010) Oligonucleotide microarray with a minimal number of probes for the detection and identification of thirteen genera of plant viruses. J Virol Methods 167(1):53–60
Zhang Y, Singh K, Kaur R, Qiu W (2011) Association of a novel DNA virus with the grapevine vein-clearing and decline syndrome. Phytopathology 101:1081–1090
Zhao L, Feng CH, Li BQ, Hao XA, Liu H, Wu YF, Wang QC (2014) Rapid detection of apple stem grooving virus by reverse transcription loop-mediated isothermal amplification. J Plant Pathol 96(2):407–409
Zhao LM, Li G, Gao Y, Zhu Y, Liu J, Zhu X (2015) Reverse transcription loop-mediated isothermal amplification assay for detecting tomato chlorosis virus. J Virol Methods 213:93–97
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer Science+Business Media Singapore
About this chapter
Cite this chapter
Selvarajan, R., Balasubramanian, V. (2016). Cutting-Edge Technologies for Detection of Plant Viruses in Vegetatively Propagated Crop Plants. In: Gaur, R., Petrov, N., Patil, B., Stoyanova, M. (eds) Plant Viruses: Evolution and Management. Springer, Singapore. https://doi.org/10.1007/978-981-10-1406-2_5
Download citation
DOI: https://doi.org/10.1007/978-981-10-1406-2_5
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-1405-5
Online ISBN: 978-981-10-1406-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)