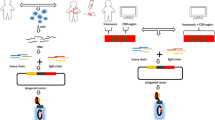
Abstract
Antibody phage display is an in vitro technology to generate recombinant antibodies. In particular for pathogens like viruses or toxins, antibody phage display is an alternative to hybridoma technology, since it circumvents the limitations of the immune system. Phage display allows the generation of human antibodies from naive antibody gene libraries when either immunized patients are not available or immunization is not ethically feasible. This technology also allows the construction of immune libraries to select in vivo affinity matured antibodies if immunized patients or animals are available.
In this review, we describe the generation of human and human-like antibodies from naive antibody gene libraries and antibodies from immune antibody gene libraries. Furthermore, we give an overview about phage display derived recombinant antibodies against viruses and toxins for diagnostics and therapy.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Antibodies are essential molecules as tools for basic research [24], diagnostics [78] and for therapy [101]. First polyclonal antibodies were produced as serum in horses [126]. A milestone in antibody generation was the development of hybridoma technology which allows the production of monoclonal antibodies [62]. But the hybridoma technology has drawbacks like limited number of candidates, possible instability of the aneuploid cell lines [94], inability to provide antibodies against highly conserved antigens and most of all its limited application to generate human antibodies [130]. The hybridoma technology thus essentially allows the isolation of murine antibodies which have a broad detection range and can be applied for diagnostic or research uses. However, their therapeutic applications are limited because repeated administration of murine antibodies can cause human anti-mouse antibody reaction (HAMA), reducing antibody half-life and have severe side effects including anaphylactic shock [26]. A strategy to circumvent these problems is antibody humanization or the use of transgenic animals where the original antibody gene repertoire is replaced with a human gene repertoire [34, 57, 70, 85]. A further strategy is the human hybridoma technology resulting in human antibodies [23, 42], but this technology is subjected – as the murine hybridoma technology – to the limitations of the immune system.
A technology which circumvents the limitation of the immune system is antibody phage display . This technology is completely independent of any immune system by an in vitro selection process. The display method most commonly used today is based on the work of Georg P. Smith on filamentous phage, which infect E. coli [111]. The selection process is called “panning”, referring to the gold digger’s tool [92].
Phage display technology was further developed 1990/1991 for antibodies in three places in parallel: Heidelberg (Germany), Cambridge (UK) and La Jolla (USA) [12, 14, 22, 76]. Antibody phage display is described in detail by Tomszak et al. “Selection of recombinant human antibodies” in this book or by [35]. In brief, antibody fragments are displayed on the surface of M13 phage and the corresponding antibody gene is packaged in the phage particle, mainly using a phagemid. The most common antibody formats used for antibody phage display are the single chain fragment variable (scFv ) [52, 107, 124] or fragment antigen binding (Fabs) [29, 45]. Other antibody formats used for phage display are single chain Fabs (scFab), human VH domains (dAbs), the variable domains of camel heavy chains (VHHs) and immunoglobulins of sharks (IgNARs) [46, 51, 83, 84, 88, 89]. Specific antibody phage can be selected from antibody gene libraries by “panning” on the desired target. Finally, monoclonal antibody phage or monoclonal soluble antibodies can be identified e.g. by ELISA . The antibody fragment genes can be recloned in any other antibody format, e.g. scFv-Fc or IgG [30, 35, 45, 52, 56].
The phage display libraries are built from immunized or non-immunized lymphocytes donors or from synthetic repertoires. Libraries using the naive IgM repertoire of a donor, which corresponds to the primary immune response, or synthetic antibody sequences are summarized as “single-pot” or universal libraries. These universal libraries are designed to isolate antibody fragments against every possible antigen, at least in theory [30, 129]. This library type was used to generate antibodies against viruses [61] or toxins [103]. Immune libraries are constructed from patients or immunized humans or animals. Also from these immune libraries, human antibodies were selected against viruses [105] or toxins [96]. Further details about antibody gene libraries are given in the chapter 3 “Selection of Recombinant Human Antibodies ” by Tomszak et al. or by [35].
To date, 44 antibodies and antibody conjugates were approved by EMA and/or FDA (status summer 2015) (http://www.imgt.org/mAb-DB/index) and about 350 antibodies were under development in 2013 [102]. Most approved therapeutic antibodies are for cancer and autoimmune diseases. Mechanisms of therapeutic antibodies are manifold and include neutralization of substances e.g. toxins [97] or cytokines like tumor necrosis factor (TNF) alpha [2], blocking of receptors like epidermal growth factor receptor (EGFR) [95], binding to cells and modulating the host immune system [18], or combinations of these effects [1]. To date, two recombinant antibodies are approved for the treatment of viruses and toxins. Raxibacumab is a human antibody for anthrax treatment derived from a phage display library from Cambridge Antibody Technology (now Medimmune) [75]. The antibody Palivizumab for the treatment of Respiratory syncytial virus (RSV) bronchiolitis is a classical humanised antibody [71].
An overview about recombinant antibodies derived from phage display against viruses and toxins is given in this review.
2 Recombinant Antibodies Against Viruses
Up to now a large panel of antibodies against various viruses has been generated from either naive or immune libraries using phage display technology. Panning against peptides , recombinant viral proteins, or complete virus particles has led to the identification of antibodies directed against human pathogenic viruses such as Sin nombre virus [125], Dengue virus [15, 106], Influenza virus [67, 113], VEEV [61], Norovirus [44], SARS coronavirus [114] or Hepatitis C [112] from naive antibody gene libraries. Other antibodies were selected from immune antibody gene libraries targeting e.g. WEEV [48], HIV [80, 122], SARS [59], Yellow fever virus [27] or Influenza virus [116, 119]. Even semi-synthetic libraries were used to generate antibodies specific for Influenza virus [9].
Libraries originating from different species have been successfully employed to isolate virus specific antibodies in the past. Among others, libraries were constructed from macaque [105], mouse [68], chimpanzee [39], llama [33] and human origin [120].
Most commonly virus specific antibodies have been isolated from libraries in scFv format [41, 131] but Fab libraries [132, 134] and VHH libraries [33] were also successfully used.
In the following paragraphs, we give detailed examples for antibody generation using phage display and antibody engineering against different virus groups.
Vaccinia Virus is the prototype virus in the genus of orthopoxvirus. It is a relatively large DNA virus with a genome of about 200 kbp [13]. The genus orthopoxvirus includes various species such as monkeypox virus, cowpox virus and especially variola virus which is the causative agent of smallpox in humans. Naturally occurring smallpox has been eradicated in 1977 because of a massive WHO vaccination program that begun in 1967. However, no vaccination of the civilian population is conducted nowadays and potential threat of intentional release has renewed the search for safe and effective smallpox vaccines as case fatality rates of 30 % or more among unvaccinated subjects are reported [43]. Because orthopoxviruses are highly related, it is assumed, that immunity against one poxvirus goes along with immunity against most members of the entire virus family [13, 31]. Using an immune scFv phage display library constructed from vaccinia virus immunized patients, a panel of human vaccinia specific antibodies was selected. Plaque-reduction neutralization tests revealed that seven of these antibodies neutralized vaccinia as well as cowpox virus in vitro. Five of those antibodies additionally neutralized monkeypox virus [120]. Other antibodies were generated from a Fab immune library derived from vaccinia virus immunized chimpanzee. Converted into a chimeric chimpanzee/human IgG format, two antibodies displayed high affinities to vaccinia protein B5 (Kd of 0,2 and 0,7 nM). Antibody 8AH8AL was neutralizing in vitro for vaccinia and smallpox virus and proofed to be protective in mice challenged with vaccinia virus even when administered 2 days after challenge. In this model 8AH8AL proofed to provide significantly greater protection than that of the previously isolated rat anti-B5 antibody 19C2 [20]. Vaccinia had to be used as model, because the final confirmation of protection against smallpox is not possible.
Ebola Virus and Marburg Virus, two filoviruses, cause severe hemorrhagic fever and possess high mortality of up to 90 % in humans. In addition to public health concerns associated with natural outbreaks, Ebola virus might be a potential agent of biological warfare and bio-terrorism [40]. Human antibodies directed against Ebola Virus were selected from a library originated from patients that recovered from infection in the 1995 Ebola virus outbreak in Kikwit, Democratic Republic of Congo [74]. Several antibodies against various viral proteins such as nucleoprotein, envelope glycoprotein and secreted envelope glycoprotein have been isolated in this study. One antibody (specific for envelope glycoprotein), KZ52 was neutralizing in vitro as Fab (50 % neutralization at 0.4 μg/ml) and as full IgG (90 % neutralization at 2.6 μg/ml) [73]. Follow-up studies were showing effective protection in vivo in a Guinea pig Ebola challenge model when the antibody was administered up to 1 h post viral challenge [93]. Interestingly KZ52 was not protective in macaques challenged with Ebola even if the antibody was given as a two-dose treatment with the first dose 1 day prior viral challenge and the second dose 4 days post challenge [91]. A murine scFv and two shark IgNAR V immune libraries were generated against inactivated Zaire Ebolavirus to yield various antibodies specific for the viral matrix protein VP40 and the viral nucleoprotein [40]. Interestingly, this work represents the first example of a successful targeted IgNAR V isolation from a shark immune response library.
Dengue virus (DENV), a member of the Flaviviridae family, is responsible for at least 100 million symptomatic infections each year and has developed into a major health and economic burden in over 50 countries worldwide [28, 81, 82]. It is a positive strand RNA virus with a ~11 kb genome, that compromises a single open reading frame. The four circulating serotypes of dengue virus show approximately 70 % sequence homology [81, 134]. Fab monoclonal antibodies to dengue type 4 virus were isolated from a chimpanzee immune library. Two Fabs, namely 5H2 and 5D9 neutralized DENV-4 efficiently with a titer of 0.24–0.58 μg/ml by plague reduction neutralization test [77]. Another study selected human scFv antibodies specific to dengue virus envelope protein by panning against recombinant full length envelope protein and its domain III [106]. Because DENV envelope protein is an essential molecule for virion assembly and virus entry, scFvs selected in this study were shown to exhibit inhibitory effects on DENV infection in vitro [106]. Dengue nonstructural protein 5 (NS5) is essential for viral replication and host immune response modulation, which makes it an excellent target for dengue-inhibiting antibodies. A naive human Fab-phage library was screened for NS5 specific antibody fragments using various NS5 variants from Dengue Virus serotypes 1–4 as antigens for panning and characterization [134]. Using NS5 from alternating dengue serotypes for each round of panning, this strategy resulted in the identification of two clones that are cross-reactive against all four dengue serotypes. Another study selected antibodies using phage display by panning with Dengue virus particles directly captured from supernatant of infected Vero cells. Here, highly serotype specific antibodies could be generated. From a total of nine antibodies, seven were shown to be specific to only one serotype. One Dengue-3 selected clone cross-reacted with Dengue 1, whereas another clone showed cross-reactivity with all serotypes despite being selected solely on Dengue 2 particles. Interestingly, all of the obtained antibodies recognized several strains of distinct genotypes within the corresponding serotype [15]. Panning against dengue envelope protein identified an antibody (C9) from a mouse/human chimeric Fab library that crossreacts with DENV1-3 and neutralizes DENV2 in cell-based assays after conversion into full length IgG [81]. Besides scFv and Fab, also variable domain heavy-chain antibodies (VHH antibodies) were selected using phage display technology. After four rounds of panning on recombinant DENV 2 NS1 protein, 20 positive clones were selected. Affinity measurements with NS1 revealed a KD value of 2,79 × 10−8 M for the best VHH antibody P2 [33].
Venezuelan equine encephalitis virus (VEEV), an alphavirus of the Togoviridae family, causes equine epidemics but can also cause encephalitis in humans [58, 128]. Because this virus is classified as Category B agent by the Centers for Disease Control and Prevention (CDC), much research has been done to generate neutralizing antibodies against it. Antibodies were generated from an immune library from human donors targeting both VEEV envelope glycoproteins E1 and E2 [49]. The isolated Fabs L1A7 and F5 were neutralizing in vitro, with F5 being 300 times more effective than L1A7. Subsequently, F5 was converted into full IgG format and was employed to generate neutralization-escape variants of VEEV for epitope mapping. Within another study, an immune macaque library was used to generate human-like antibodies [105]. One of these antibodies, scFv -Fc ToR67-3B4, was protective in mice when it was administered 6 h post viral challenge with VEEV Trinidiad strains. Here, 80–100 % of mice survived a lethal viral dose. However, scFv-Fc ToR67-3B4 was not able to neutralize Trinidad strain but other VEEV strains in vitro, showing that neutralization is not mandatory for an in vivo protective antibody [105]. Another study describes the selection of antibodies from a human naive scFv gene library using complete, active VEEV particles as antigen. Here, specific detection of the VEEV strains TC83, H12/93 and 230 by the selected antibodies was proven. Remarkably, none of the selected scFv phage clones did show any cross-reactivity with Alphavirus species of the Eastern equine encephalitis virus (EEEV) and Western equine encephalitis virus (WEEV) antigenic complex or with Chikungunya virus (CHIKV), making them ideal tools for the immunological detection and diagnosis of Alphavirus species [61]. Two different scFv antibody libraries were constructed from WEEV immunized macaques. Subcloned as scFv-Fc, three antibodies from these libraries specifically bound WEEV in ELISA with little or no cross-reactivity with other alphaviruses and were found to be neutralizing in vitro. In this study, the first antibodies against WEEV, that were shown to be neutralizing in vitro, were developed. About 1 ng/ml of the best antibody (ToR69-3A2) neutralized 50 % of 5 × 104 TCID50/ml WEEV [48].
An overview about recombinant antibodies generated by phage display against viruses is given in Table 1.
3 Antibodies Against Toxins
Several toxins are classified by the Center for Disease Control and Prevention (CDC) as category A or B agents that are relevant for diagnostics and therapeutics . They can easily be disseminated and result in high or moderate mortality rates [36]. Here, the antibody phage display presents a powerful tool for antibody selection and allows the isolation of neutralizing antibodies against complete active toxins or special domains from different human naive antibody gene libraries with high diversity [5, 54, 86]. For the isolation of high-affinity antibodies against specific targets, animals are immunized with toxoids, non-toxic subunits or selected toxin domains. Alternatively, human material from immunized patients can be used for construction of immune libraries [17, 79, 96, 97].
In the following paragraphs, we give detailed examples for antibody generation using phage display and antibody engineering against different toxins .
So far, antibody phage display was successfully used for antibody selection against a panel of toxins classified as category A agents, such as from Clostridium botulinum (botulism) [16, 25, 53, 79] and Bacillus anthracis (anthrax) [97] and also against different category B agents, such as staphylococcal enterotoxin B [66, 110] and ricin toxin from Ricinus communis [6, 96]. An example for a high-risk microorganism that produces the most toxic substances known with the highest risk of potential use as bioweapons is the Gram-positive, anaerobic, spore-forming bacterium Clostridium botulinum and other Clostridium subspecies. They are secreting eight different serotypes (A–H) of botulinum neurotoxin (BoNT). Five serotypes (A, B, E, rarely F and only one case of H) are known to cause human botulism, a disease characterized by flaccid muscle paralysis requiring intensive hospital care and passive immunization [8, 11]. Especially, serotype A is recognized as the most toxic substance known with LD50 values (lethal dose) of 1 ng/kg by intravenous and subcutaneous routes and 3 ng/kg by the pulmonary route [38]. BoNTs are composed of a disulfide bond-linked 50 kDa light chain and a 100 kDa heavy chain. The heavy chain contains two functional domains (Hc and Hn) that are responsible for toxin uptake into nerve cells by receptor-mediated endocytosis and for the translocation of the light chain across the membrane into the neuronal cytosol. Whereas the catalytic domain of the light chain is responsible for the BoNT toxicity. The current approach for treatment of botulism includes the application of human anti-botulism immunoglobulins, such as Baby botulism immune globulin (BabyBIG), or equine anti-toxin serum. But the human serum stock of BabyBIG is limited and the equine anti-toxin may cause hypersensitivity and serum sickness. Here, antibody phage display provides a technology to generate toxin-neutralizing antibodies against each serotype. For instance, a macaque immune library was used to isolate neutralizing scFv with nm affinities against the light chain of BoNT/A [16, 79], but also antibodies against the heavy chain or other relevant serotypes of BoNT are of therapeutical interest. Phage display technology was also used for isolation of single domain antibodies (VHH) after immunization of a llama with a cocktail of seven BoNT toxoids (A–F) [25]. Another approach was the generation of a human antibody gene library after inducing a BoNT/A-specific immune response by in vitro immunization [53]. Furthermore, antibody phage display was used to generate antibodies against other clostridial toxins such as from Clostridium tetani or Clostridium difficile [17, 50].
Anthrax, another serious infectious disease is caused by Bacillus anthracis, an aerobic, Gram-positive, spore-forming bacterium that is found in soils around the world. Bacillus anthracis secrets two toxins , the lethal toxin (LT) and the edema toxin (ET) [69]. Both toxins are composed of two subunits. The LT consists of the lethal factor (LF) and the protective antigen (PA), the ET is formed by the edema factor (EF) and PA. It was demonstrated that only LT has an essential role in the pathogenesis of anthrax [55]. The subunit PA is the basis of current vaccines and induces the generation of neutralizing antibodies . In combination with antibiotics, commercial monoclonal antibodies against PA, such as Raxibacumab, are commonly used for treatment [65]. In 2012, the FDA approved Raxibacumab to treat inhalational anthrax. Due to security issues the use of anti-PA antibodies alone is questionable, since PA could be modified and lose the recognized epitopes while retaining biological activity. An alternative to anti-PA antibodies are antibodies targeting the LF, such as 2LF, which was isolated from an immune library via antibody phage display technology [97]. A combination of an anti-PA antibody with an anti-LF antibody could lead to a synergistic effect and improve the efficacy of the therapy .
An example for bacterial toxins classified as category B agent is staphylococcus enterotoxin B from Staphylococcus aureus. This bacteria are a potential causative agent for food-borne illness and produces 21 types of staphylococcal enterotoxins that cause symptoms of food poisoning including abdominal cramps, vomiting and diarrhea [90, 118]. The staphylococcal enterotoxin B (SEB), a single polypeptide of 28 kDa, is the most potent toxin secreted by S. aureus. As a superantigen, it stimulates T cells and leads to an overproduction of cytokines, causing clinical symptoms such as fever, hypertension and in some cases death. Phage display was used to generate recombinant antibodies from a murine immune library [110] and to identify the epitope of a SEB specific monoclonal antibody using a peptide phage library [123]. Furthermore, a human monoclonal antibody against SEB was isolated from a synthetic human antibody gene library that inhibited SEB binding to MHCII [66].
The phage display technology was also used to isolate antibodies against ricin. Ricin is a 61 kDa glycoprotein from the castor bean plant (Ricinus communis), which consists of two distinct subunits (RTA and RTB). RTB is a galactose- and N-acetylgalactosamine specific lectin which binds to specific sugar residues on the cell surface, allowing internalization of the toxin by endocytosis [87], whereas RTA has an RNA N-glycosidase activity that irreversibly inactivates eukaryotic ribosomes resulting in inhibition of protein synthesis [32]. Ricin is also classified as category B agent by CDC. Human-like antibodies were selected by phage display from a macaque immunized with RTA. One antibody , 43RCA, had a picomolar affinity and neutralized the biological activity of ricin in vitro [96]. Furthermore, neutralizing antibodies with high affinities were selected from a llama immune library [6].
In addition to the different toxins that are classified by the CDC as category A or B agents, the number of relevant toxins is almost endless. In addition, different animals are known to produce high potential toxins containing a complex composition. For example, one of them is Tityus serrulatus, known as brazilian yellow scorpion, the most dangerous scorpion in Brazil. The major toxic component in the venom of T. serrulatus is the gamma-toxin , a polypeptide of 61 amino acid residues [60, 99]. Here, a neutralizing antibody was isolated from a human library via phage display and was protecting in mice [5]. The same procedure was used for Bothrops jararacussu, a venomous pit viper species endemic in south america. By using a human antibody gene library different antibodies were selected that inhibit the phospholipase activity of the venom in vitro and reduce the myotoxicity in vivo [104]. Toxins are also produced by marine organism. An example is the tetrodoxin (TTX) of the toxic puffer fish. Here, scFv were selected from a human naive antibody gene library neutralizing the TTX activity [21].
An overview about recombinant antibodies generated by phage display against toxins is given in Table 2.
4 Conclusion
Antibody phage display allows the generation of (human/camel/macaque/shark…) antibodies from mainly two types of sources: immune and naive libraries. Immune libraries should be preferred when immunized animals or convalescent patients are available, offering the chance to directly isolate neutralizing and/or protective antibodies. If immunization is not possible or ethically not feasible, naive antibody gene libraries are an alternative. In an such approach, the antibody generation process is not limited by the immune system. Antibody phage display has provided a panel of antibodies useful for diagnostics and therapy against toxins and viruses .
References
Adams GP, Weiner LM (2005) Monoclonal antibody therapy of cancer. Nat Biotechnol 23:1147–1157. doi:10.1038/nbt1137
Alonso-Ruiz A, Pijoan JI, Ansuategui E et al (2008) Tumor necrosis factor alpha drugs in rheumatoid arthritis: systematic review and metaanalysis of efficacy and safety. BMC Musculoskelet Disord 9:52. doi:1471-2474-9-52
Amersdorfer P, Wong C, Chen S et al (1997) Molecular characterization of murine humoral immune response to botulinum neurotoxin type A binding domain as assessed by using phage antibody libraries. Infect Immun 65:3743–3752
Amersdorfer P, Wong C, Smith T et al (2002) Genetic and immunological comparison of anti-botulinum type A antibodies from immune and non-immune human phage libraries. Vaccine 20:1640–1648. doi:10.1016/S0264-410X(01)00482-0
Amaro I, Riaño-Umbarila L, Becerril B, Possani LD (2011) Isolation and characterization of a human antibody fragment specific for Ts1 toxin from Tityus serrulatus scorpion. Immunol Lett 139:73–79. doi:10.1016/j.imlet.2011.05.002
Anderson GP, Liu JL, Hale ML et al (2008) Development of antiricin single domain antibodies toward detection and therapeutic reagents. Anal Chem 80:9604–9611. doi:10.1021/ac8019398
Arakawa M, Yamashiro T, Uechi G et al (2007) Construction of human Fab (gamma1/kappa) library and identification of human monoclonal Fab possessing neutralizing potency against Japanese encephalitis virus. Microbiol Immunol 51:617–625
Arnon SS, Schechter R, Inglesby TV et al (2001) Botulinum toxin as a biological weapon: medical and public health management. JAMA J Am Med Assoc 285:1059–1070
Avnir Y, Tallarico AS, Zhu Q et al (2014) Molecular signatures of hemagglutinin stem-directed heterosubtypic human neutralizing antibodies against influenza A viruses. PLoS Pathog 10:e1004103. doi:10.1371/journal.ppat.1004103
Bakherad H, Mousavi Gargari SL, Rasooli I et al (2013) In vivo neutralization of botulinum neurotoxins serotype E with heavy-chain camelid antibodies (VHH). Mol Biotechnol 55:159–167. doi:10.1007/s12033-013-9669-1
Barash JR, Arnon SS (2014) A novel strain of clostridium botulinum that produces type B and type H botulinum toxins. J Infect Dis 209:183–191. doi:10.1093/infdis/jit449
Barbas CF, Kang AS, Lerner RA, Benkovic SJ (1991) Assembly of combinatorial antibody libraries on phage surfaces: the gene III site. Proc Natl Acad Sci U S A 88:7978–7982. doi:1896445
Berhanu A, Wilson RL, Kirkwood-Watts DL et al (2008) Vaccination of BALB/c mice with escherichia coli-expressed vaccinia virus proteins A27L, B5R, and D8L protects mice from lethal vaccinia virus challenge. J Virol 82:3517–3529. doi:10.1128/JVI.01854-07
Breitling F, Dübel S, Seehaus T et al (1991) A surface expression vector for antibody screening. Gene 104:147–153
Cabezas S, Rojas G, Pavon A et al (2008) Selection of phage-displayed human antibody fragments on dengue virus particles captured by a monoclonal antibody: application to the four serotypes. J Virol Methods 147:235–243. doi:10.1016/j.jviromet.2007.09.001
Chahboun S, Hust M, Liu Y et al (2011) Isolation of a nanomolar scFv inhibiting the endopeptidase activity of botulinum toxin A, by single-round panning of an immune phage-displayed library of macaque origin. BMC Biotechnol 11:113. doi:10.1186/1472-6750-11-113
Chassagne S, Laffly E, Drouet E et al (2004) A high-affinity macaque antibody Fab with human-like framework regions obtained from a small phage display immune library. Mol Immunol 41:539–546. doi:10.1016/j.molimm.2004.03.040
Chatenoud L, Bluestone JA (2007) CD3-specific antibodies: a portal to the treatment of autoimmunity. Nat Rev Immunol 7:622–632. doi:nri2134
Chen Z, Chumakov K, Dragunsky E et al (2011) Chimpanzee-human monoclonal antibodies for treatment of chronic poliovirus excretors and emergency postexposure prophylaxis. J Virol 85:4354–4362. doi:10.1128/JVI.02553-10
Chen Z, Earl P, Americo J et al (2006) Chimpanzee/human mAbs to vaccinia virus B5 protein neutralize vaccinia and smallpox viruses and protect mice against vaccinia virus. Proc Natl Acad Sci U S A 103:1882–1887. doi:10.1073/pnas.0510598103
Chulanetra M, Bangphoomi K, Sookrung N et al (2012) Human ScFv that block sodium ion channel activity of tetrodotoxin. Toxicon 59:272–282. doi:10.1016/j.toxicon.2011.11.012
Clackson T, Hoogenboom HR, Griffiths AD, Winter G (1991) Making antibody fragments using phage display libraries. Nature 352:624–628. doi:1907718
Cole SP, Campling BG, Atlaw T et al (1984) Human monoclonal antibodies. Mol Cell Biochem 62:109–120
Colwill K, Gräslund S (2011) A roadmap to generate renewable protein binders to the human proteome. Nat Methods 8:551–558. doi:10.1038/nmeth.1607
Conway JO, Sherwood LJ, Collazo MT et al (2010) Llama single domain antibodies specific for the 7 botulinum neurotoxin serotypes as heptaplex immunoreagents. PLoS One 5:e8818. doi:10.1371/journal.pone.0008818
Courtenay-Luck NS, Epenetos AA, Moore R et al (1986) Development of primary and secondary immune responses to mouse monoclonal antibodies used in the diagnosis and therapy of malignant neoplasms. Cancer Res 46:6489–6493. doi:2430699
Daffis S, Kontermann RE, Korimbocus J et al (2005) Antibody responses against wild-type yellow fever virus and the 17D vaccine strain: characterization with human monoclonal antibody fragments and neutralization escape variants. Virology 337:262–272. doi:10.1016/j.virol.2005.04.031
De Alwis R, Smith SA, Olivarez NP et al (2012) Identification of human neutralizing antibodies that bind to complex epitopes on dengue virions. Proc Natl Acad Sci U S A 109:7439–7444. doi:10.1073/pnas.1200566109
De Haard HJ, van Neer N, Reurs A et al (1999) A large non-immunized human Fab fragment phage library that permits rapid isolation and kinetic analysis of high affinity antibodies. J Biol Chem 274:18218–18230. doi:10373423
Dübel S, Stoevesandt O, Taussig MJ, Hust M (2010) Generating recombinant antibodies to the complete human proteome. Trends Biotechnol 28:333–339. doi:10.1016/j.tibtech.2010.05.001
Earl PL, Americo JL, Wyatt LS et al (2004) Immunogenicity of a highly attenuated MVA smallpox vaccine and protection against monkeypox. Nature 428:182–185. doi:10.1038/nature02331
Endo Y, Mitsui K, Motizuki M, Tsurugi K (1987) The mechanism of action of ricin and related toxic lectins on eukaryotic ribosomes. The site and the characteristics of the modification in 28 S ribosomal RNA caused by the toxins. J Biol Chem 262:5908–5912
Fatima A, Wang H, Kang K et al (2014) Development of VHH antibodies against dengue virus type 2 NS1 and comparison with monoclonal antibodies for use in immunological diagnosis. PLoS One 9:e95263. doi:10.1371/journal.pone.0095263
Fishwild DM, O’Donnell SL, Bengoechea T et al (1996) High-avidity human IgG kappa monoclonal antibodies from a novel strain of minilocus transgenic mice. Nat Biotechnol 14:845–851. doi:9631008
Frenzel A, Kügler J, Wilke S et al (2014) Construction of human antibody gene libraries and selection of antibodies by phage display. Methods Mol Biol 1060:215–243. doi:10.1007/978-1-62703-586-6_12
Froude JW, Stiles B, Pelat T, Thullier P (2011) Antibodies for biodefense. MAbs 3:517–527. doi:10.4161/mabs.3.6.17621
Funayama JC, Pucca MB, Roncolato EC et al (2012) Production of human antibody fragments binding to melittin and phospholipase A2 in Africanised bee venom: minimising venom toxicity. Basic Clin Pharmacol Toxicol 110:290–297. doi:10.1111/j.1742-7843.2011.00821.x
Gill DM (1982) Bacterial toxins: a table of lethal amounts. Microbiol Rev 46:86–94
Goncalvez AP, Chien C-H, Tubthong K et al (2008) Humanized monoclonal antibodies derived from chimpanzee Fabs protect against Japanese encephalitis virus in vitro and in vivo. J Virol 82:7009–7021. doi:10.1128/JVI.00291-08
Goodchild SA, Dooley H, Schoepp RJ et al (2011) Isolation and characterisation of Ebolavirus-specific recombinant antibody fragments from murine and shark immune libraries. Mol Immunol 48:2027–2037. doi:10.1016/j.molimm.2011.06.437
Gould LH, Sui J, Foellmer H et al (2005) Protective and therapeutic capacity of human single-chain Fv-Fc fusion proteins against West Nile virus. J Virol 79:14606–14613. doi:10.1128/JVI.79.23.14606-14613.2005
Hammond PW (2010) Accessing the human repertoire for broadly neutralizing HIV antibodies. MAbs 2:157–164
Henderson DA, Inglesby TV, Bartlett JG et al (1999) Smallpox as a biological weapon: medical and public health management. Working Group on Civilian Biodefense. JAMA 281:2127–2137
Higo-Moriguchi K, Shirato H, Someya Y et al (2014) Isolation of cross-reactive human monoclonal antibodies that prevent binding of human noroviruses to histo-blood group antigens. J Med Virol 86:558–567. doi:10.1002/jmv.23734
Hoet RM, Cohen EH, Kent RB et al (2005) Generation of high-affinity human antibodies by combining donor-derived and synthetic complementarity-determining-region diversity. Nat Biotechnol 23:344–348. doi:nbt1067
Holt LJ, Herring C, Jespers LS et al (2003) Domain antibodies: proteins for therapy. Trends Biotechnol 21:484–490
Houimel M (2014) The analysis of VH and VL genes repertoires of Fab library built from peripheral B cells of human rabies virus vaccinated donors. Hum Immunol 75:745–755. doi:10.1016/j.humimm.2014.05.005
Hülseweh B, Rülker T, Pelat T et al (2014) Human-like antibodies neutralizing Western equine encephalitis virus. MAbs 6:717–726. doi:10.4161/mabs.28170
Hunt AR, Frederickson S, Maruyama T et al (2010) The first human epitope map of the alphaviral E1 and E2 proteins reveals a new E2 epitope with significant virus neutralizing activity. PLoS Negl Trop Dis 4:e739. doi:10.1371/journal.pntd.0000739
Hussack G, Arbabi-Ghahroudi M, van Faassen H et al (2011) Neutralization of clostridium difficile toxin A with single-domain antibodies targeting the cell receptor binding domain. J Biol Chem 286:8961–8976. doi:10.1074/jbc.M110.198754
Hust M, Jostock T, Menzel C et al (2007) Single chain Fab (scFab) fragment. BMC Biotechnol 7:14
Hust M, Meyer T, Voedisch B et al (2011) A human scFv antibody generation pipeline for proteome research. J Biotechnol 152:159–170. doi:10.1016/j.jbiotec.2010.09.945
Hu W-G, Jager S, Chau D et al (2010) Generation of a recombinant full-length human antibody binding to botulinum neurotoxin A. Appl Biochem Biotechnol 160:1206–1216. doi:10.1007/s12010-009-8657-1
Indrawattana N, Sookrung N, Kulkeaw K et al (2010) Human monoclonal ScFv that inhibits cellular entry and metalloprotease activity of tetanus neurotoxin. Asian Pac J Allergy Immunol 28:85–93
Inglesby TV, O’Toole T, Henderson DA et al (2002) Anthrax as a biological weapon, 2002: updated recommendations for management. JAMA J Am Med Assoc 287:2236–2252
Jäger V, Büssow K, Wagner A et al (2013) High level transient production of recombinant antibodies and antibody fusion proteins in HEK293 cells. BMC Biotechnol 13:52. doi:10.1186/1472-6750-13-52
Jakobovits A (1995) Production of fully human antibodies by transgenic mice. Curr Opin Biotechnol 6:561–566. doi:7579668
Johnson KM, Martin DH (1974) Venezuelan equine encephalitis. Adv Vet Sci Comp Med 18:79–116
Kang X, Yang B-A, Hu Y et al (2006) Human neutralizing Fab molecules against severe acute respiratory syndrome coronavirus generated by phage display. Clin Vaccine Immunol CVI 13:953–957. doi:10.1128/CVI.00037-06
Kirsch GE, Skattebøl A, Possani LD, Brown AM (1989) Modification of Na channel gating by an alpha scorpion toxin from Tityus serrulatus. J Gen Physiol 93:67–83
Kirsch M, Hülseweh B, Nacke C et al (2008) Development of human antibody fragments using antibody phage display for the detection and diagnosis of Venezuelan equine encephalitis virus (VEEV). BMC Biotechnol 8:66. doi:1472-6750-8-66
Köhler G, Milstein C (1975) Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 256:495–497. doi:1172191
Kramer RA, Marissen WE, Goudsmit J et al (2005) The human antibody repertoire specific for rabies virus glycoprotein as selected from immune libraries. Eur J Immunol 35:2131–2145. doi:10.1002/eji.200526134
Kulkeaw K, Sakolvaree Y, Srimanote P et al (2009) Human monoclonal ScFv neutralize lethal Thai cobra, Naja kaouthia, neurotoxin. J Proteomics 72:270–282. doi:10.1016/j.jprot.2008.12.007
Kummerfeldt CE (2014) Raxibacumab: potential role in the treatment of inhalational anthrax. Infect Drug Resist 7:101–109. doi:10.2147/IDR.S47305
Larkin EA, Stiles BG, Ulrich RG (2010) Inhibition of toxic shock by human monoclonal antibodies against staphylococcal enterotoxin B. PLoS One 5:e13253. doi:10.1371/journal.pone.0013253
Lim APC, Chan CEZ, Wong SKK et al (2008) Neutralizing human monoclonal antibody against H5N1 influenza HA selected from a Fab-phage display library. Virol J. doi:10.1186/1743-422X-5-130
Liu H, Zheng X, Shi X et al (2014) Selection and characterization of single-chain recombinant antibodies against infectious haematopoietic necrosis virus from mouse phage display library. J Virol Methods 205C:61–67. doi:10.1016/j.jviromet.2014.04.008
Liu S, Moayeri M, Leppla SH (2014) Anthrax lethal and edema toxins in anthrax pathogenesis. Trends Microbiol 22:317–325. doi:10.1016/j.tim.2014.02.012
Lonberg N, Huszar D (1995) Human antibodies from transgenic mice. Int Rev Immunol 13:65–93. doi:7494109
Malley R, DeVincenzo J, Ramilo O et al (1998) Reduction of respiratory syncytial virus (RSV) in tracheal aspirates in intubated infants by use of humanized monoclonal antibody to RSV F protein. J Infect Dis 178:1555–1561
Maneewatch S, Thanongsaksrikul J, Songserm T et al (2009) Human single-chain antibodies that neutralize homologous and heterologous strains and clades of influenza A virus subtype H5N1. Antivir Ther 14:221–230
Maruyama T, Parren PW, Sanchez A et al (1999) Recombinant human monoclonal antibodies to Ebola virus. J Infect Dis 179(Suppl 1):S235–S239. doi:10.1086/514280
Maruyama T, Rodriguez LL, Jahrling PB et al (1999) Ebola virus can be effectively neutralized by antibody produced in natural human infection. J Virol 73:6024–6030
Mazumdar S (2009) Raxibacumab. MAbs 1:531–538
McCafferty J, Griffiths AD, Winter G, Chiswell DJ (1990) Phage antibodies: filamentous phage displaying antibody variable domains. Nature 348:552–554
Men R, Yamashiro T, Goncalvez AP et al (2004) Identification of chimpanzee Fab fragments by repertoire cloning and production of a full-length humanized immunoglobulin G1 antibody that is highly efficient for neutralization of dengue type 4 virus. J Virol 78:4665–4674
Meyer T, Stratmann-Selke J, Meens J et al (2011) Isolation of scFv fragments specific to OmpD of Salmonella Typhimurium. Vet Microbiol 147:162–169. doi:10.1016/j.vetmic.2010.06.023
Miethe S, Rasetti-Escargueil C, Liu Y et al (2014) Development of neutralizing scFv-Fc against botulinum neurotoxin A light chain from a macaque immune library. MAbs 6:446–459. doi:10.4161/mabs.27773
Mohammadzadeh S, Rajabibazl M, Fourozandeh M et al (2014) Production of recombinant scFv against p24 of human immunodeficiency virus type 1 by phage display technology. Monoclon Antib Immunodiagn Immunother 33:28–33. doi:10.1089/mab.2013.0059
Moreland NJ, Susanto P, Lim E et al (2012) Phage display approaches for the isolation of monoclonal antibodies against dengue virus envelope domain III from human and mouse derived libraries. Int J Mol Sci 13:2618–2635. doi:10.3390/ijms13032618
Moreland NJ, Tay MYF, Lim E et al (2010) High affinity human antibody fragments to dengue virus non-structural protein 3. PLoS Negl Trop Dis 4:e881. doi:10.1371/journal.pntd.0000881
Muyldermans S (2001) Single domain camel antibodies: current status. J Biotechnol 74:277–302
Muyldermans S, Baral TN, Retamozzo VC et al (2009) Camelid immunoglobulins and nanobody technology. Vet Immunol Immunopathol 128:178–183. doi:10.1016/j.vetimm.2008.10.299
Nelson AL, Dhimolea E, Reichert JM (2010) Development trends for human monoclonal antibody therapeutics. Nat Rev Drug Discov 9:767–774. doi:10.1038/nrd3229
Neri P, Shigemori N, Hamada-Tsutsumi S et al (2011) Single chain variable fragment antibodies against Shiga toxins isolated from a human antibody phage display library. Vaccine 29:5340–5346. doi:10.1016/j.vaccine.2011.05.093
Nicolson GL, Lacorbiere M, Hunter TR (1975) Mechanism of cell entry and toxicity of an affinity- purified lectin from Ricinus communis and its differential effects on normal and virus-transformed fibroblasts. Cancer Res 35:144–155
Nuttall SD, Humberstone KS, Krishnan UV et al (2004) Selection and affinity maturation of IgNAR variable domains targeting Plasmodium falciparum AMA1. Proteins 55:187–197. doi:10.1002/prot.20005
Nuttall SD, Krishnan UV, Hattarki M et al (2001) Isolation of the new antigen receptor from wobbegong sharks, and use as a scaffold for the display of protein loop libraries. Mol Immunol 38:313–326
Ono HK, Omoe K, Imanishi K et al (2008) Identification and characterization of two novel staphylococcal enterotoxins, types S and T. Infect Immun 76:4999–5005. doi:10.1128/IAI.00045-08
Oswald WB, Geisbert TW, Davis KJ et al (2007) Neutralizing antibody fails to impact the course of Ebola virus infection in monkeys. PLoS Pathog 3:e9. doi:10.1371/journal.ppat.0030009
Parmley SF, Smith GP (1988) Antibody-selectable filamentous fd phage vectors: affinity purification of target genes. Gene 73:305–318
Parren PWHI, Geisbert TW, Maruyama T et al (2002) Pre- and postexposure prophylaxis of Ebola virus infection in an animal model by passive transfer of a neutralizing human antibody. J Virol 76:6408–6412
Pauza ME, Rehmann JA, LeBien TW (1993) Unusual patterns of immunoglobulin gene rearrangement and expression during human B cell ontogeny: human B cells can simultaneously express cell surface kappa and lambda light chains. J Exp Med 178:139–149
Peeters M, Price T, Van Laethem J-L (2009) Anti-epidermal growth factor receptor monotherapy in the treatment of metastatic colorectal cancer: where are we today? Oncologist 14:29–39. doi:10.1634/theoncologist.2008-0167
Pelat T, Hust M, Hale M et al (2009) Isolation of a human-like antibody fragment (scFv) that neutralizes ricin biological activity. BMC Biotechnol 9:60. doi:10.1186/1472-6750-9-60
Pelat T, Hust M, Laffly E et al (2007) High-affinity, human antibody-like antibody fragment (single-chain variable fragment) neutralizing the lethal factor (LF) of Bacillus anthracis by inhibiting protective antigen-LF complex formation. Antimicrob Agents Chemother 51:2758–2764
Pereira SS, Moreira-Dill LS, Morais MSS et al (2014) Novel camelid antibody fragments targeting recombinant nucleoprotein of Araucaria hantavirus: a prototype for an early diagnosis of hantavirus pulmonary syndrome. PLoS One 9:e108067. doi:10.1371/journal.pone.0108067
Possani LD, Martin BM, Svendsen I et al (1985) Scorpion toxins from Centruroides noxius and Tityus serrulatus. Primary structures and sequence comparison by metric analysis. Biochem J 229:739–750
Ray K, Embleton MJ, Jailkhani BL et al (2001) Selection of single chain variable fragments (scFv) against the glycoprotein antigen of the rabies virus from a human synthetic scFv phage display library and their fusion with the Fc region of human IgG1. Clin Exp Immunol 125:94–101
Reichert JM (2012) Marketed therapeutic antibodies compendium. MAbs 4:413–415. doi:10.4161/mabs.19931
Reichert JM (2013) Which are the antibodies to watch in 2013? MAbs 5:1–4. doi:10.4161/mabs.22976
Riaño-Umbarila L, Juárez-González VR, Olamendi-Portugal T et al (2005) A strategy for the generation of specific human antibodies by directed evolution and phage display. An example of a single-chain antibody fragment that neutralizes a major component of scorpion venom. FEBS J 272:2591–2601. doi:10.1111/j.1742-4658.2005.04687.x
Roncolato EC, Pucca MB, Funayama JC et al (2013) Human antibody fragments specific for Bothrops jararacussu venom reduce the toxicity of other Bothrops sp. venoms. J Immunotoxicol 10:160–168. doi:10.3109/1547691X.2012.703253
Rülker T, Voß L, Thullier P et al (2012) Isolation and characterisation of a human-like antibody fragment (scFv) that inactivates VEEV in vitro and in vivo. PLoS One 7:e37242. doi:10.1371/journal.pone.0037242
Saokaew N, Poungpair O, Panya A et al (2014) Human monoclonal single-chain antibodies specific to dengue virus envelope protein. Lett Appl Microbiol 58:270–277. doi:10.1111/lam.12186
Schofield DJ, Pope AR, Clementel V et al (2007) Application of phage display to high throughput antibody generation and characterization. Genome Biol 8:R254. doi:10.1186/gb-2007-8-11-r254
Schofield DJ, Satterfield W, Emerson SU, Purcell RH (2002) Four chimpanzee monoclonal antibodies isolated by phage display neutralize hepatitis a virus. Virology 292:127–136. doi:10.1006/viro.2001.1252
Shingarova LN, Tikunova NV, Iun TE et al (2007) Recombinant full-size human antibody to Ebola virus. Bioorg Khim 33:598–605
Singh PK, Agrawal R, Kamboj DV et al (2010) Construction of a single-chain variable-fragment antibody against the superantigen Staphylococcal enterotoxin B. Appl Environ Microbiol 76:8184–8191. doi:10.1128/AEM.01441-10
Smith GP (1985) Filamentous fusion phage: novel expression vectors that display cloned antigens on the virion surface. Science 228:1315–1317
Songsivilai S, Dharakul T (1998) Genetically engineered single-chain Fvs of human immunoglobulin against hepatitis C virus nucleocapsid protein derived from universal phage display library. Asian Pac J Allergy Immunol 16:31–41
Sui J, Hwang WC, Perez S et al (2009) Structural and functional bases for broad-spectrum neutralization of avian and human influenza A viruses. Nat Struct Mol Biol 16:265–273. doi:10.1038/nsmb.1566
Sui J, Li W, Murakami A et al (2004) Potent neutralization of severe acute respiratory syndrome (SARS) coronavirus by a human mAb to S1 protein that blocks receptor association. Proc Natl Acad Sci U S A 101:2536–2541
Sun L, Chen Z, Yu L et al (2012) Generation and characterization of neutralizing human recombinant antibodies against antigenic site II of rabies virus glycoprotein. Appl Microbiol Biotechnol 96:357–366. doi:10.1007/s00253-012-4171-4
Sun L, Lu X, Li C et al (2009) Generation, characterization and epitope mapping of two neutralizing and protective human recombinant antibodies against influenza A H5N1 viruses. PLoS One 4:e5476. doi:10.1371/journal.pone.0005476
Thanongsaksrikul J, Srimanote P, Maneewatch S et al (2010) A V H H that neutralizes the zinc metalloproteinase activity of botulinum neurotoxin type A. J Biol Chem 285:9657–9666. doi:10.1074/jbc.M109.073163
Thomas DY, Jarraud S, Lemercier B et al (2006) Staphylococcal enterotoxin-like toxins U2 and V, two new staphylococcal superantigens arising from recombination within the enterotoxin gene cluster. Infect Immun 74:4724–4734. doi:10.1128/IAI.00132-06
Throsby M, van den Brink E, Jongeneelen M et al (2008) Heterosubtypic neutralizing monoclonal antibodies cross-protective against H5N1 and H1N1 recovered from human IgM+ memory B cells. PLoS One 3:e3942. doi:10.1371/journal.pone.0003942
Tikunova N, Dubrovskaya V, Morozova V et al (2012) The neutralizing human recombinant antibodies to pathogenic Orthopoxviruses derived from a phage display immune library. Virus Res 163:141–150. doi:10.1016/j.virusres.2011.09.008
Tremblay JM, Mukherjee J, Leysath CE et al (2013) A single VHH-based toxin-neutralizing agent and an effector antibody protect mice against challenge with Shiga toxins 1 and 2. Infect Immun 81:4592–4603. doi:10.1128/IAI.01033-13
Trott M, Weiβ S, Antoni S et al (2014) Functional characterization of two scFv-Fc antibodies from an HIV controller selected on soluble HIV-1 Env complexes: a neutralizing V3- and a trimer-specific gp41 antibody. PLoS One 9:e97478. doi:10.1371/journal.pone.0097478
Urushibata Y, Itoh K, Ohshima M, Seto Y (2010) Generation of Fab fragment-like molecular recognition proteins against staphylococcal enterotoxin B by phage display technology. Clin Vaccine Immunol CVI 17:1708–1717. doi:10.1128/CVI.00229-10
Vaughan TJ, Williams AJ, Pritchard K et al (1996) Human antibodies with sub-nanomolar affinities isolated from a large non-immunized phage display library. Nat Biotechnol 14:309–314. doi:9630891
Velappan N, Martinez JS, Valero R et al (2007) Selection and characterization of scFv antibodies against the Sin Nombre hantavirus nucleocapsid protein. J Immunol Methods 321:60–69. doi:10.1016/j.jim.2007.01.011
Von Behring E, Kitasato S (1890) Über das Zustandekommen der Diphtherie-Immunität und der Tetanus-Immunität bei Thieren. Dtsch Med Wochenzeitschrift 16:1113–1114
Wang Y, Zhang X, Zhang C et al (2012) Isolation of single chain variable fragment (scFv) specific for Cry1C toxin from human single fold scFv libraries. Toxicon 60:1290–1297. doi:10.1016/j.toxicon.2012.08.014
Weaver SC, Ferro C, Barrera R et al (2004) Venezuelan equine encephalitis. Annu Rev Entomol 49:141–174. doi:10.1146/annurev.ento.49.061802.123422
Winter G, Griffiths AD, Hawkins RE, Hoogenboom HR (1994) Making antibodies by phage display technology. Annu Rev Immunol 12:433–455. doi:8011287
Winter G, Milstein C (1991) Man-made antibodies. Nature 349:293–299. doi:1987490
Wu J, Zeng X-Q, Zhang H-B et al (2014) Novel phage display-derived H5N1-specific scFvs with potential use in rapid avian flu diagnosis. J Microbiol Biotechnol 24:704–713
Wyrzucki A, Dreyfus C, Kohler I et al (2014) Alternative recognition of the conserved stem epitope in influenza A virus hemagglutinin by a VH3-30-encoded heterosubtypic antibody. J Virol 88:7083–7092. doi:10.1128/JVI.00178-14
Yang Z, Schmidt D, Liu W et al (2014) A novel multivalent, single-domain antibody targeting TcdA and TcdB prevents fulminant Clostridium difficile infection in mice. J Infect Dis 210:964–972. doi:10.1093/infdis/jiu196
Zhao Y, Moreland NJ, Tay MYF et al (2014) Identification and molecular characterization of human antibody fragments specific for dengue NS5 protein. Virus Res 179:225–230. doi:10.1016/j.virusres.2013.11.010
Zhu Z, Dimitrov AS, Bossart KN et al (2006) Potent neutralization of Hendra and Nipah viruses by human monoclonal antibodies. J Virol 80:891–899. doi:10.1128/JVI.80.2.891-899.2006
Acknowledgments
We acknowledge funding from the European Community’s Seventh Framework Program (FP7/2007–2013) under agreement no. 241832 granted to the AntiBotABE project (http://www.antibotabe.com) and funding from Federal State of Lower Saxony, Niedersächsisches Vorab (VWZN2889).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Unkauf, T., Miethe, S., Fühner, V., Schirrmann, T., Frenzel, A., Hust, M. (2016). Generation of Recombinant Antibodies Against Toxins and Viruses by Phage Display for Diagnostics and Therapy. In: Böldicke, T. (eds) Protein Targeting Compounds. Advances in Experimental Medicine and Biology, vol 917. Springer, Cham. https://doi.org/10.1007/978-3-319-32805-8_4
Download citation
DOI: https://doi.org/10.1007/978-3-319-32805-8_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-32804-1
Online ISBN: 978-3-319-32805-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)