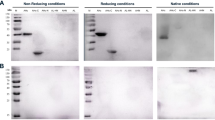
Abstract
Ingestion of botulinum neurotoxin (BoNT) results in botulism, a severe and frequent fatal disease known in the world. Current treatments rely on antitoxins, such as equine antitoxin and human botulism immunoglobulin. In some cases, side effects have been reported, including early anaphylactic shock and late serum sickness. Thus, diagnosis and treatment measure of BoNT are necessary and crucial. In the present study, a single-domain variable heavy-chain (VHH) antibody fragment was obtained from an immune dromedary phage display library against the putative binding domain of botulinum neurotoxin E (BoNT/E), a non-toxic 50-kDa fragment. The characteristics of nanobody VHH include excellent production, superior heat stability and specific binding capacity to soluble antigen without cross-reaction to other relevant or irrelevant antigens. A total of 150 ng/Kg of nanobody entirely neutralized 3LD50 of the BoNT/E in an in vivo challenge of the mice. This phenomenon indicates BoNT/E toxin neutralizing capacity of the produced nanobody. These results also suggest possession of unique properties by the nanobody applicable in diagnostics or therapeutic purposes.








Similar content being viewed by others
References
Arbabi Ghahroudi, M., Desmyter, A., Wyns, L., Hamers, R., & Muyldermans, S. (1997). Selection and identification of single domain antibody fragments from camel heavy-chain antibodies. FEBS Letters, 414, 521–526.
Basiri, M., Mousavi, S. L., Basiri, H., & Rasooli, I. (2010). An epitopic approach to designing and characterization of a multiple antigenic polypeptide against Botulinum neurotoxins A and E. World Journal of Microbiology & Biotechnology, 26, 1659–1666.
Beatty, J. D., Beatty, B. G., & Vlahos, W. G. (1987). Measurement of monoclonal antibody affinity by non-competitive enzyme immunoassay. Journal of Immunological Methods, 100, 173–179.
Behar, G., Siberil, S., Groulet, A., Chames, P., Pugniere, M., Boix, C., et al. (2008). Isolation and characterization of anti-FcγRIII (CD16) llama single-domain antibodies that activate natural killer cells. Protein Engineering, Design & Selection, 21, 1–10.
Black, R. E., & Gunn, R. A. (1980). Hypersensitivity reactions associated with botulinal antitoxin. The American journal of medicine, 69, 567–570.
Conrath, K. E., Lauwereys, M., Galleni, M., Matagne, A., Frere, J. M., Kinne, J., et al. (2001). β-Lactamase inhibitors derived from single-domain antibody fragments elicited in the camelidae. Antimicrobial Agents and Chemotherapy, 45, 2807.
Devaux, C., Moreau, E., Goyffon, M., Rochat, H., & Billiald, P. (2001). Construction and functional evaluation of a single chain antibody fragment that neutralizes toxin AahI from the venom of the scorpion Androctonus australis hector. European Journal of Biochemistry, 268, 694–702.
Dolly, J. O., Black, J., Williams, R. S., & Melling, J. (1984). Acceptors for botulinum neurotoxin reside on motor nerve terminals and mediate its internalization.
Dong, J., Thompson, A. A., Fan, Y., Lou, J., Conrad, F., Ho, M., et al. (2010). A single-domain llama antibody potently inhibits the enzymatic activity of botulinum neurotoxin by binding to the non-catalytic [alpha]-exosite binding region. Journal of Molecular Biology, 397, 1106–1118.
Doyle, P. J., Arbabi-Ghahroudi, M., Gaudette, N., Furzer, G., Savard, M. E., Gleddie, S., et al. (2008). Cloning, expression, and characterization of a single-domain antibody fragment with affinity for 15-acetyl-deoxynivalenol. Molecular Immunology, 45, 3703–3713.
Dressler, D., & Adib Saberi, F. (2005). Botulinum toxin: Mechanisms of action. European Neurology, 53, 3–9.
Dumoulin, M., Conrath, K., Van Meirhaeghe, A., Meersman, F., Heremans, K., Frenken, L. G. J., et al. (2002). Single-domain antibody fragments with high conformational stability. Protein Science, 11, 500–515.
Ebrahimizadeh, W., Mousavi Gargari, S., Rajabibazl, M., Safaee Ardekani, L., Zare, H. & Bakherad, H. (2012). Isolation and characterization of protective anti-LPS nanobody against V. cholerae O1 recognizing Inaba and Ogawa serotypes. Applied Microbiology and Biotechnology 1–10.
Frenken, L. G. J., van der Linden, R. H. J., Hermans, P. W. J. J., Bos, J. W., Ruuls, R. C., de Geus, B., et al. (2000). Isolation of antigen specific llama VHH antibody fragments and their high level secretion by Saccharomyces cerevisiae. Journal of Biotechnology, 78, 11–21.
Gargari, S. L. M., Rasooli, I., Valipour, E., Basiri, M., Nazarian, S., Amani, J., & Farhadi, N. (2011). Immunogenic and protective potentials of recombinant receptor binding domain and a C-terminal fragment of Clostridium botulinum neurotoxin type E. Iranian Journal of Biotechnology (IJB) 9.
Gessler, F., Hampe, K., Schmidt, M., & Bohnel, H. (2006). Immunomagnetic beads assay for the detection of botulinum neurotoxin types C and D. Diagnostic Microbiology and Infectious Disease, 56, 225–232.
Gill, D. M. (1982). Bacterial toxins: A table of lethal amounts. Microbiology and Molecular Biology Reviews, 46, 86.
Hamers-Casterman, C., Atarhouch, T., Muyldermans, S., Robinson, G., Hammers, C., Songa, E. B., et al. (1993). Naturally occurring antibodies devoid of light chains. Nature, 363, 446–448.
Harmsen, M. M., & De Haard, H. J. (2007). Properties, production, and applications of camelid single-domain antibody fragments. Applied Microbiology and Biotechnology, 77, 13–22.
Harmsen, M. M., Van Solt, C. B., Van Zijderveld-van Bemmel, A., Niewold, T. A., & Van Zijderveld, F. G. (2006). Selection and optimization of proteolytically stable llama single-domain antibody fragments for oral immunotherapy. Applied Microbiology and Biotechnology, 72, 544–551.
Hayhurst, A., & Georgiou, G. (2001). High-throughput antibody isolation. Current Opinion in Chemical Biology, 5, 683–689.
Hibbs, R. G., Weber, J. T., Corwin, A., Allos, B. M., El Rehim, M. S. A., El Sharkawy, S., et al. (1996). Experience with the use of an investigational F (ab’) 2 heptavalent botulism immune globulin of equine origin during an outbreak of type E botulism in Egypt. Clinical Infectious Diseases, 23, 337.
Hmila, I., Abdallah, R., Saerens, D., Benlasfar, Z., Conrath, K., Ayeb, M. E., et al. (2008). VHH, bivalent domains and chimeric Heavy chain-only antibodies with high neutralizing efficacy for scorpion toxin AahI’. Molecular Immunology, 45, 3847–3856.
Jones, R. G. A., Corbel, M. J., & Sesardic, D. (2006). A review of WHO International Standards for botulinum antitoxins. Biologicals, 34, 223–226.
Kastelic, D., Frkovic-Grazio, S., Baty, D., Truan, G., Komel, R., & Pompon, D. (2009). A single-step procedure of recombinant library construction for the selection of efficiently produced llama VH binders directed against cancer markers. Journal of Immunological Methods, 350, 54–62.
Lacy, D. B., Tepp, W., Cohen, A. C., DasGupta, B. R., & Stevens, R. C. (1998). Crystal structure of botulinum neurotoxin type A and implications for toxicity. Nature Structural & Molecular Biology, 5, 898–902.
Ladenson, R. C., Crimmins, D. L., Landt, Y., & Ladenson, J. H. (2006). Isolation and characterization of a thermally stable recombinant anti-caffeine heavy-chain antibody fragment. Analytical Chemistry, 78, 4501–4508.
Lalli, G., Herreros, J., Osborne, S. L., Montecucco, C., Rossetto, O., & Schiavo, G. (1999). Functional characterisation of tetanus and botulinum neurotoxins binding domains. Journal of Cell Science, 112, 2715–2724.
Ligler, F. S., Taitt, C. R., Shriver-Lake, L. C., Sapsford, K. E., Shubin, Y., & Golden, J. P. (2003). Array biosensor for detection of toxins. Analytical and Bioanalytical Chemistry, 377, 469–477.
Mahrhold, S., Rummel, A., Bigalke, H., Davletov, B., & Binz, T. (2006). The synaptic vesicle protein 2C mediates the uptake of botulinum neurotoxin A into phrenic nerves. FEBS Letters, 580, 2011–2014.
Maynard, J., & Georgiou, G. (2000). Antibody engineering. Annual Review of Biomedical Engineering, 2, 339–376.
Mousli, M., Devaux, C., Rochat, H., Goyffon, M., & Billiald, P. (1999). A recombinant single-chain antibody fragment that neutralizes toxin II from the venom of the scorpion Androctonus australis hector. FEBS Letters, 442, 183–188.
Muyldermans, S. (2001). Single domain camel antibodies: current status. Reviews in Molecular Biotechnology, 74, 277–302.
Muyldermans, S., Atarhouch, T., Saldanha, J., Barbosa, J., & Hamers, R. (1994). Sequence and structure of VH domain from naturally occurring camel heavy chain immunoglobulins lacking light chains. Protein Engineering, 7, 1129.
Nguyen, V. K., Hamers, R., Wyns, L., & Muyldermans, S. (2000). Camel heavy-chain antibodies: diverse germline VHH and specific mechanisms enlarge the antigen-binding repertoire. The EMBO Journal, 19, 921–930.
Rivera, V. R., Gamez, F. J., Keener, W. K., White, J. A., & Poli, M. A. (2006). Rapid detection of Clostridium botulinum toxins A, B, E, and F in clinical samples, selected food matrices, and buffer using paramagnetic bead-based electrochemiluminescence detection. Analytical Biochemistry, 353, 248–256.
Sambrook, J., & Russell, D. W. (2001). Molecular cloning: a laboratory manual. CSHL Press.
Sharma, S. K., & Whiting, R. C. (2005). Methods for detection of Clostridium botulinum toxin in foods. Journal of Food Protection&# 174, 68, 1256–1263.
Sharma, S. K., Ferreira, J. L., Eblen, B. S., & Whiting, R. C. (2006). Detection of type A, B, E, and F Clostridium botulinum neurotoxins in foods by using an amplified enzyme-linked immunosorbent assay with digoxigenin-labeled antibodies. Applied and Environmental Microbiology, 72, 1231–1238.
Simpson, L. L. (1980). Kinetic studies on the interaction between botulinum toxin type A and the cholinergic neuromuscular junction. Journal of Pharmacology and Experimental Therapeutics, 212, 16.
Simpson, L. L. (1981). The origin, structure, and pharmacological activity of botulinum toxin. Pharmacological Reviews, 33, 155–188.
Simpson, L. L. (1990). The study of clostridial and related toxins. The search for unique mechanisms and common denominators. Journal de physiologie, 84, 143.
Swain, M. D., Anderson, G. P., Zabetakis, D., Bernstein, R. D., Liu, J. L., Sherwood, L. J., et al. (2010). Llama-derived single-domain antibodies for the detection of botulinum A neurotoxin. Analytical and Bioanalytical Chemistry, 398, 339–348.
Tremblay, J. M., Kuo, C. L., Abeijon, C., Sepulveda, J., Oyler, G., Hu, X., et al. (2010). Camelid single domain antibodies (VHHs) as neuronal cell intrabody binding agents and inhibitors of Clostridium botulinum neurotoxin (BoNT) proteases. Toxicon, 56, 990–998.
Vermeer, A. W. P., & Norde, W. (2000). The thermal stability of immunoglobulin: unfolding and aggregation of a multi-domain protein. Biophysical Journal, 78, 394–404.
Vu, K. B., Ghahroudi, M. A., Wyns, L., & Muyldermans, S. (1997). Comparison of llama VH sequences from conventional and heavy chain antibodies. Molecular Immunology, 34, 1121–1131.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bakherad, H., Mousavi Gargari, S.L., Rasooli, I. et al. In Vivo Neutralization of Botulinum Neurotoxins Serotype E with Heavy-chain Camelid Antibodies (VHH). Mol Biotechnol 55, 159–167 (2013). https://doi.org/10.1007/s12033-013-9669-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-013-9669-1