Abstract
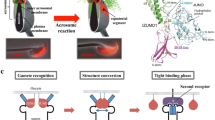
The acrosome reaction (AR) is a universal requisite for sperm–egg fusion. However, whereas through the animal kingdom fusion of spermatozoa with the egg plasma membrane occurs via the inner acrosomal membrane exposed after the AR, in eutherian mammals, gamete fusion takes place through a specialized region of the acrosome known as the equatorial segment (ES) which becomes fusogenic only after the AR is completed. This chapter focuses on the different molecular mechanisms involved in the acquisition of the fusogenicity of the ES after the AR. We provide an update of the knowledge about the proteins proposed to have a role in this process either by modifying cytoskeletal and/or membrane molecules or by relocalizing to the ES after the AR to subsequently participate in gamete fusion.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Alhadeff JA, Khunsook S, Choowongkomon K et al (1999) Characterization of human semen alpha-L-fucosidases. Mol Hum Reprod 5:809–815
Anderson DH, Michaelson JS, Johnson PM (1989) Trophoblast/leukocyte-common antigen is expressed by human testicular germ cells and appears on the surface of acrosome-reacted sperm. Biol Reprod 41:285–293
Anderson DJ, Abbott AF, Jack RM (1993) The role of complement component C3b and its receptors in sperm-oocyte interaction. Proc Natl Acad Sci USA 90:10051–10055
Aoyama T, Osaki Y, Suzumori K et al (2001) Involvement of μ-calpain in human sperm capacitation for fertilization. Am J Reprod Immunol 45:12–20
Avilés M, Abascal I, Martinez-Menarguez JA et al (1996) Immunocytochemical localization and biochemical characterization of a novel plasma membrane-associated, neutral ph optimum alpha-L-fucosidase from rat testis and epididymal spermatozoa. Biochem J 318:821–831
Baba T, Azuma S, Kashiwabara S et al (1994) Sperm from mice carrying a targeted mutation of the acrosin gene can penetrate the oocyte zona pellucida and effect fertilization. J Biol Chem 269:31845–31849
Bastián Y, Roa-Espitia AL, Mújica A et al (2010) Calpain modulates capacitation and acrosome reaction through cleavage of the spectrin cytoskeleton. Reproduction 140:673–684
Bedford JM (2004) Enigmas of mammalian gamete form and function. Biol Rev Camb Philos Soc 79:429–460
Bedford JM (2014) Singular features of fertilization and their impact on the male reproductive system in eutherian mammals. Reproduction 147:43–52
Bedford JM, Moore HD, Franklin LE (1979) Significance of the equatorial segment of the acrosome of the spermatozoon in eutherian mammals. Exp Cell Res 119:119–126
Ben-Aharon I, Brown PR, Etkovitz N et al (2005) The expression of calpain 1 and calpain 2 in spermatogenic cells and spermatozoa of the mouse. Reproduction 129:435–442
Bianchi E, Doe B, Goulding D et al (2014) Juno is the egg Izumo receptor and is essential for mammalian fertilization. Nature 508:483–487
Blobel C, Wolsberg T, Turck C et al (1992) A potential fusion peptide and an integrin ligand domain in a protein active in sperm-egg fusion. Nature 356:248–252
Boldt J, Howe AM, Parkerson JB et al (1989) Carbohydrate involvement in sperm-egg fusion in mice. Biol Reprod 40:887–896
Brukman NG, Miyata H, Torres P et al (2016) Fertilization defects in sperm from Cysteine-rich secretory protein 2 (Crisp2) knockout mice: implications for fertility disorders. Mol Hum Reprod 22:240–251
Busso D, Cohen DJ, Hayashi M et al (2005) Human testicular protein TPX1/CRISP-2: localization in spermatozoa, fate alter capacitation and relevance for gamete interaction. Mol Hum Reprod 11:299–305
Busso D, Goldweic NM, Hayashi M et al (2007) Evidence for the involvement of testicular protein CRISP2 in mouse sperm-egg fusion. Biol Reprod 76:701–708
Cameo M, Blaquier J (1976) Androgen-controlled specific proteins in rat epididymis. J Endocrinol 69:317–324
Cervoni F, Oglesby TJ, Adams EM et al (1992) Identification and characterization of membrane cofactor protein of human spermatozoa. J Immunol 148:1431–1437
Chalbi M, Barraud-Lange V, Ravaux B et al (2014) Binding of sperm protein Izumo1 and its egg receptor Juno drives Cd9 accumulation in the intercellular contact area prior to fusion during mammalian fertilization. Development 141:3732–3739
Cho C, Bunch DO, Faure JE et al (1998) Fertilization defects in sperm from mice lacking fertilin β. Science 281:1857–1859
Cohen DJ, Rochwerger L, Ellerman DA et al (2000a) Relationship between the association of rat epididymal protein DE with spermatozoa and the behaviour and function of the protein. Mol Reprod Dev 56:180–188
Cohen DJ, Ellerman DA, Cuasnicú PS (2000b) Mammalian sperm-egg fusion: evidence that epididymal protein DE plays a role in mouse gamete fusion. Biol Reprod 63:462–468
Cohen DJ, Ellerman DA, Busso D et al (2001) Evidence that human epididymal protein ARP plays a role in gamete fusion through complementary sites on the surface of the human egg. Biol Reprod 65:1000–1005
Da Ros VG, Maldera JA, Willis WD et al (2008) Impaired sperm fertilizing ability in mice lacking Cysteine-RIch Secretory Protein 1 (CRISP1). Dev Biol 320:12–18
Ellerman DA, Cohen DJ, Weigel Muñoz M et al (2010) Immunologic behavior of human cysteine-rich secretory protein 1 (hCRISP1) in primates: prospects for immunocontraception. Fertil Steril 93:2551–2556
Eto K, Huet C, Tarui T et al (2002) Functional classification of ADAMs based on a conserved motif for binding to integrin α9β1 implications for sperm–egg binding and other cell interactions. J Biol Chem 277:17804–17810
Evans JP (2002) The molecular basis of sperm-oocyte membrane interactions during mammalian fertilization. Hum Reprod Update 8:297–311
Fenichel P, Hsi BL, Farahifar D et al (1989) Evaluation of the human sperm acrosome reaction using a monoclonal antibody, GB24, and fluorescence-activated cell sorter. J Reprod Fertil 87:699–704
Flesch FM, Gadella BM (2000) Dynamics of the mammalian sperm plasma membrane in the process of fertilization. Biochim Biophys Acta 1469:197–235
Foster JA, Gerton GL (1996) Autoantigen 1 of the guinea pig sperm acrosome is the homologue of mouse Tpx-1 and human TPX1 and is a member of the cysteine-rich secretory protein (CRISP) family. Mol Reprod Dev 44:221–229
Franco SJ, Huttenlocher A (2005) Regulating cell migration: calpains make the cut. J Cell Sci 118:3829–3838
Fujihara Y, Murakami M, Inoue N et al (2010) Sperm equatorial segment protein 1, SPESP1, is required for fully fertile sperm in mouse. J Cell Sci 123:1531–1536
Gadella BM, Tsai PS, Boerke A et al (2008) Sperm head membrane reorganisation during capacitation. Int J Dev Biol 52:473–480
Garberi JC, Kohane AC, Cameo MS et al (1979) Isolation and characterization of specific rat epididymal proteins. Mol Cell Endocrinol 13:73–82
Garberi JC, Fontana JD, Blaquier JA (1982) Carbohydrate composition of specific rat epididymal protein. Int J Androl 5:619–626
Gibbs GM, Bianco DM, Jamsai D et al (2007) Cysteine-rich secretory protein 2 binds to mitogen-activated protein kinase kinase kinase 11 in mouse sperm. Biol Reprod 77:108–114
Goll DE, Thompson VF, Li H et al (2003) The calpain system. Physiol Rev 83:731–801
Haendler B, Kratzschmar J, Theuring F et al (1993) Transcripts for cysteine-rich secretory protein-1 (CRISP-1; DE/AEG) and the novel related CRISP-3 are expressed under androgen control in the mouse salivary gland. Endocrinology 133:192–198
Hancock LW, Raab LS, Aronson NN Jr (1993) Synthesis and processing of rat sperm-associated alpha-L-fucosidase. Biol Reprod 48:1228–1238
Hao J, Chen M, Ji S et al (2014) Equatorin is not essential for acrosome biogenesis but is required for the acrosome reaction. Biochem Biophys Res Commun 444:537–542
Hardy DM, Huang TTF, Driscoll WJ et al (1988) Purification and characterization of the primary acrosomal autoantigen of guinea pig epididymal spermatozoa. Biol Reprod 38:423–437
Hardy DM, Oda MN, Friend DS et al (1991) A mechanism for differential release of acrosomal enzymes during the acrosome reaction. Biochem J 275:759–766
Hayashi M, Fujimoto S, Takano H et al (1996) Characterization of a human glycoprotein with potential role in sperm-egg fusion: cDNA cloning, immunohistochemical localization, and chromosomal assignment of the gene (AEGL1). Genomics 32:367–374
Herrero MB, Mandal A, Digilio LC et al (2005) Mouse SLLP1, a sperm lysozyme-like protein involved in sperm-egg binding and fertilization. Dev Biol 284:126–142
Inoue N, Ikawa M, Nakanishi T et al (2003) Disruption of mouse CD46 causes an accelerated spontaneous acrosome reaction in sperm. Mol Cell Biol 23:2614–2622
Inoue N, Ikawa M, Isotani A et al (2005) The immunoglobulin superfamily protein Izumo is required for sperm to fuse with eggs. Nature 434:234–238
Jauhiainen A, Vanha-Perttula T (1986) alpha-L-Fucosidase in the reproductive organs and seminal plasma of the bull. Biochim Biophys Acta 880:91–95
Johnson PM, Clift LE, Andrlíková P et al (2007) Rapid sperm acrosome reaction in the absence of acrosomal CD46 expression in promiscuous field mice (Apodemus). Reproduction 134:739–747
Kim KS, Foster JA, Gerton GL (2001) Differential release of guinea pig sperm acrosomal components during exocytosis. Biol Reprod 64:148–156
Kohane AC, Cameo MS, Piñeiro L et al (1980a) Distribution and site of production of specific proteins in the rat epididymis. Biol Reprod 23:181–187
Kohane AC, Gonzalez Echeverria F, Piñeiro L et al (1980b) Interaction of proteins of epididymal origin with spermatozoa. Biol Reprod 23:737–742
Kratzschmar J, Haendler B, Eberspaecher U et al (1996) The human cysteine-rich secretory protein (CRISP) family. Primary structure and tissue distribution of CRISP-1, CRISP-2 and CRISP-3. Eur J Biochem 236:827–836
Kwak KB, Kambayashi J, Kang MS et al (1993) Cell-penetrating inhibitors of calpain block both membrane fusion and filamin cleavage in chick embryonic myoblasts. FEBS Lett 323:151–154
Lebart MC, Benyamin Y (2006) Calpain involvement in the remodeling of cytoskeletal anchorage complexes. FEBS J 273:3415–3426
Lv ZM, Wang M, Xu C (2010) Antifertility characteristics of the N-terminal region of mouse equatorial segment protein. Anat Rec (Hoboken) 293:171–181
Mandal A, Klotz KL, Shetty J et al (2003) SLLP1, a unique, intra-acrosomal, non-bacteriolytic, c lysozyme-like protein of human spermatozoa. Biol Reprod 68:1525–1537
Mizuki N, Kasahara M (1992) Mouse submandibular glands express an androgen-regulated transcript encoding an acidic epididymal glycoprotein-like molecule. Mol Cell Endocrinol 89:25–32
Molinari M, Carafoli E (1997) Calpain: a cytosolic proteinase active at the membranes. J Membr Biol 156:1–8
Munoz MW, Ernesto JI, Bluguermann C et al (2012) Evaluation of testicular sperm CRISP2 as a potential target for contraception. J Androl 33:1360–1370
Nimlamool W, Bean BS, Lowe-Krentz LJ (2013) Human sperm CRISP2 is released from the acrosome during the acrosome reaction and re-associates at the equatorial segment. Mol Reprod Dev 80:488–502
Nishimura H, Cho C, Branciforte DR et al (2001) Analysis of loss of adhesive function in sperm lacking cyritestin or fertilin β. Dev Biol 233:204–213
O’Bryan MK, Loveland KL, Herszfeld D et al (1998) Identification of a rat testis-specific gene encoding a potential rat outer dense fibre protein. Mol Reprod Dev 50:313–322
O’Bryan MK, Sebire K, Meinhardt A et al (2001) Tpx-1 is a component of the outer dense fibers and acrosome of rat spermatozoa. Mol Reprod Dev 58:116–125
Okabe M (2015) Mechanisms of fertilization elucidated by gene-manipulated animals. Asian J Androl 17:646–652
Okabe M, Adachi T, Takada K et al (1987) Capacitation-related changes in antigen distribution on mouse sperm heads and its relation to fertilization rate in vitro. J Reprod Immunol 11:91–100
Okabe M, Nagira M, Kawai Y et al (1990) A human sperm antigen possibly involved in binding and/or fusion with zona-free hamster eggs. Fertil Steril 54:1121–1126
Okabe M, Ying X, Nagira M et al (1992) Homology of an acrosome-reacted sperm-specific antigen to CD46. J Pharmacobiodyn 15:455–459
Park CK, Hwang IS, Cheong HT et al (2002) Effect of a fertilization-promoting peptide on the fertilizing ability and glycosidase activity in vitro of frozen-thawed spermatozoa in the pig. Anim Reprod Sci 72:83–94
Pastén K, Bastián Y, Roa-Espitia AL et al (2014) ADAM15 participates in fertilization through a physical interaction with acrogranin. Reproduction 148:623–634
Pasten-Hidalgo K, Hernandez-Rivas R, Roa-Espitia AL et al (2008) Presence, processing, and localization of mouse ADAM15 during sperm maturation and the role of its disintegrin domain during sperm-egg binding. Reproduction 136:41–51
Phopin K, Nimlamool W, Bartlett MJ et al (2012) Distribution, crypticity, stability and localization of alpha-L-fucosidase of mouse cauda epididymal sperm. Mol Reprod Dev 3:208–217
Phopin K, Nimlamool W, Lowe-Krentz LJ et al (2013) Roles of mouse sperm-associated alpha-L-fucosidases in fertilization. Mol Reprod Dev 80:273–285
Roberts KP, Wamstad JA, Ensrud KM et al (2003) Inhibition of capacitation-associated tyrosine phosphorylation signaling in rat sperm by epididymal protein Crisp1. Biol Reprod 69:572–581
Rochwerger L, Cuasnicu PS (1992) Redistribution of a rat sperm epididymal glycoprotein after in vivo and in vitro capacitation. Mol Reprod Dev 31:34–41
Rochwerger L, Cohen DJ, Cuasnicú PS (1992) Mammalian sperm-egg fusion: the rat egg has complementary sites for a sperm protein that mediates gamete fusion. Dev Biol 153:83–90
Rojas FJ, Moretti-Rojas I (2000) Involvement of the calcium-specific protease, calpain, in the fertilizing capacity of human spermatozoa. Int J Androl 23:163–168
Rojas FJ, Brush M, Moretti-Rojas I (1999) Calpain-calpastatin: a novel, complete calcium-dependent protease system in human spermatozoa. Mol Hum Reprod 5:520–526
Sachdev M, Mandal A, Mulders S et al (2012) Oocyte specific oolemmal SAS1B involved in sperm binding through intra-acrosomal SLLP1 during fertilization. Dev Biol 363:40–51
Satouh Y, Inoue N, Ikawa M et al (2012) Visualization of the moment of mouse sperm-egg fusion and dynamic localization of IZUMO1. J Cell Sci 125:4985–4990
Schollmeyer JE (1986) Identification of calpain II in porcine sperm. Biol Reprod 34:721–731
Seya T, Turner JR, Atkinson JP (1986) Purification and characterization of a membrane protein (gp45-70) that is a cofactor for cleavage of C3b and C4b. J Exp Med 163:837–855
Seya T, Hara T, Matsumoto M et al (1993) Membrane cofactor protein (MCP, CD46) in seminal plasma and on spermatozoa in normal and “sterile” subjects. Eur J Immunol 23:1322–1327
Shamsadin R, Adham IM, Nayernia K et al (1999) Male mice deficient for germ-cell cyritestin are infertile. Biol Reprod 61:1445–1451
Simpson KL, Holmes CH (1994) Differential expression of complement regulatory proteins decay-accelerating factor (CD55), membrane cofactor protein (CD46) and CD59 during human spermatogenesis. Immunology 81:452–461
Sivashanmugam P, Richardson RT, Hall S et al (1999) Cloning and characterization of an androgen-dependent acidic epididymal glycoprotein/CRISP1-like protein from the monkey. J Androl 20:384–393
Sosnik J, Miranda PV, Spiridonov NA et al (2009) Tssk6 is required for Izumo relocalization and gamete fusion in the mouse. J Cell Sci 122:2741–2749
Spiridonov NA, Wong L, Zerfas PM et al (2005) Identification and characterization of SSTK, a serine/threonine protein kinase essential for male fertility. Mol Cell Biol 25:4250–4261
Srivastava PN, Farooqui AA, Gould KG (1981) Studies on hydrolytic enzymes of chimpanzee semen. Biol Reprod 25:363–369
Takano H, Yanagimachi R, Urch UA (1993) Evidence that acrosin activity is important for the development of fusibility of mammalian spermatozoa with the oolemma: inhibitor studies using the golden hamster. Zygote 1:79–91
Tomes CN (2015) The proteins of exocytosis: lessons from the sperm model. Biochem J 465:359–370
Topfer-Petersen E, Friess AE, Stoffel M et al (1990) Boar sperm membranes antigens. II. Reorganization of an integral membrane antigen during capacitation and acrosome reaction. Histochemistry 93:491–495
Toshimori K, Tanii I, Araki S et al (1992) Characterization of the antigen recognized by a monoclonal antibody MN9: unique transport pathway to the equatorial segment of sperm head during spermiogenesis. Cell Tissue Res 270:459–468
Toshimori K, Saxena DK, Tanii I et al (1998) An MN9 antigenic molecule, equatorin, is required for successful sperm-oocyte fusion in mice. Biol Reprod 59:22–29
Venditti JJ, Donigan KA, Bean BS (2007) Crypticity and functional distribution of the membrane associated alpha-L-fucosidase of human sperm. Mol Reprod Dev 74:758–766
Venditti JJ, Swann JM, Bean BS (2010) Hamster sperm-associated alpha-L-fucosidase functions during fertilization. Biol Reprod 82:572–579
Wolkowicz MJ, Shetty J, Westbrook A et al (2003) Equatorial segment protein defines a discrete acrosomal subcompartment persisting throughout acrosomal biogenesis. Biol Reprod 69:735–745
Wolkowicz MJ, Digilio L, Klotz K et al (2008) Equatorial segment protein (ESP) is a human alloantigen involved in sperm-egg binding and fusion. J Androl 29:272–282
Yamatoya K, Yoshida K, Ito C et al (2009) Equatorin: identification and characterization of the epitope of the MN9 antibody in the mouse. Biol Reprod 81:889–897
Yanagimachi R (1994) Mammalian fertilization. In: Knobil E, Neill JD (eds) The physiology of reproduction, 2nd edn. Raven, New York, NY, pp 189–317
Yoshida K, Ito C, Yamatoya K et al (2010) A model of the acrosome reaction progression via the acrosomal membrane-anchored protein equatorin. Reproduction 139:533–544
Yudin AI, Goldberg E, Robertson KR et al (2000) Calpain and calpastatin are located between the plasma membrane and outer acrosomal membrane of cynomolgus macaque spermatozoa. J Androl 21:721–729
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Cuasnicú, P.S., Da Ros, V.G., Weigel Muñoz, M., Cohen, D.J. (2016). Acrosome Reaction as a Preparation for Gamete Fusion. In: Buffone, M. (eds) Sperm Acrosome Biogenesis and Function During Fertilization. Advances in Anatomy, Embryology and Cell Biology, vol 220. Springer, Cham. https://doi.org/10.1007/978-3-319-30567-7_9
Download citation
DOI: https://doi.org/10.1007/978-3-319-30567-7_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-30565-3
Online ISBN: 978-3-319-30567-7
eBook Packages: MedicineMedicine (R0)