Abstract
The caterpillars of many Lepidoptera are neither attacked nor tended by ants but nevertheless appear to be obligately ant-associated and benefit from the enemy-free space created by ants. Obligate myrmecophiles that do not attract ants through stridulatory or chemical signaling are limited to habitats where ants are reliably present for other reasons, either among ant-attended hemipterans, on ant-plants, or around ant nests. Particularly in the tropics, obligate ant associates that passively coexist with ants are more diverse than previously recognized, including, for example, hundreds of African species in the lycaenid subfamily Poritiinae. Mutualists and parasites of ants have been reported in eleven families: Tineidae, Tortricidae, Cyclotornidae, Coleophoridae, Crambidae, Erebidae, Notodontidae, Hesperiidae, Pieridae, Lycaenidae, and Riodinidae. Altogether, myrmecophily has originated at least 30 times in Lepidoptera, and many groups may remain undiscovered. The butterfly families Lycaenidae and Riodinidae contain the vast majority of ant-associated species: larvae of at least 3841 (71%) of the ~5390 described Lycaenidae and 308 (20%) of the ~1562 described Riodinidae are known or inferred to be ant-associated, and both families possess specialized, convergently developed exocrine glands and stridulatory devices to communicate with ants. Many caterpillar-ant relationships previously characterized as mutualisms may actually be parasitic, as caterpillars can manipulate ants and ultimately exert a fitness cost. In the family Lycaenidae, highly specialized and obligate ant associations are found largely in the Old World tropics, Australia, and Southern Africa, where the stoichiometry of soil micronutrients, particularly sodium and phosphorus, climate, host plants, and geography may all selectively shape caterpillar-ant associations.


A larva of Nudina artaxidia (Erebidae) steals honeydew from a monophlebid scale insect attended by Lasius nipponensis, as described in Komatsu and Itino (2014). (Photo by Takashi Komatsu)
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
- Myrmecophily
- myrmecophile
- Lycaenidae
- Riodinidae
- Lepidoptera
- Formicidae
- Ant-plant
- Mutualism
- Symbiosis
- Specialization
- Aphytophagy
- Entomophagy
- Parasitism
- Tentacle organ
- Dorsal nectary organ
- Pore cupolae organs
- Stridulation
Introduction
Caterpillars have a fantastic array of chemical, physical, and behavioral defenses to protect themselves against ants (Borges et al. 2014; Darling et al. 2001; DeVries 1991a; Dyer 1995; Freitas 1999; Honda 1983; Peterson et al. 1987; Rostás 1657; Roux et al. 2011; Uemura et al. 2017). Larvae of diverse Lepidoptera are ignored by marauding ants foraging on their host plants, either due to chemical manipulation and camouflage (Akino et al. 2004; Eubanks et al. 1997; Portugal and Trigo 2005) or physical concealment (Bächtold and Alves-Silva 2013; Farquharson et al. 1922; Ito and Higashi 1991; Jones et al. 2002; Loeffler 1996; Sendoya and Oliveira 2017). Unharmed larvae of various butterfly and moth species are also occasionally known to live close to or within ant nests (Fiedler 1991; Kistner 1982; Lamborn et al. 1914, iNaturalist #65727498). Larvae that can survive encounters with ants and colonize ant territories, whether on host plants or inside structures built by ants, may enjoy a range of benefits including reduced competition, enemy-free space, and favorable microclimates (Atsatt 1981a; Hinton 1951; Koptur 1985; Saarinen and Daniels 2006). Passive coexistence of larvae and ants, through physical/chemical protection or signaling by larvae, may be an important prerequisite to the appearance of stable ant associations in caterpillars (DeVries 1991b; Fiedler 1991) much as in other arthropod groups (Cushing 1997; Cushing 2012; Hölldobler and Wilson 1990; Parker 2016; Stadler and Dixon 2005; Vantaux et al. 2012). Particularly in tropical tree canopies, mosaics of competing ant colonies and ant species play a major role in diversifying available host plant niches, structuring caterpillar communities and creating specialized niches for those able to coexist with them (Agassiz and Kallies 2018; Baker et al. 2016; Blüthgen and Stork 2007; Camarota et al. 2020; Dejean et al. 2017; Floren et al. 2002; Sendoya and Oliveira 2014; Seufert and Fiedler 1996; Wiens et al. 1993).
In this chapter, we provide an overview of caterpillar-ant associations. A number of recent reviews focus on ant associations in Lycaenidae and Riodinidae, including Pierce et al. (2002) and Casacci et al. (2019b). Other treatments such as Kistner (1982), Hölldobler and Wilson (1990) and Pierce (1995) have reviewed the caterpillars found in nests of social insects. However, ant associations have not been summarized and critically examined across all Lepidoptera since Hinton (1951). Many novel relationships have been uncovered in the intervening 70 years, and we discuss factors that may contribute to the phylogenetic distribution and biogeography of these unusual life histories at the end of the chapter. We have not used comparative methods to analyze potential correlates of different forms of ant association, although we plan to do so in a subsequent publication that will include additional phylogenetic and quantitative life history measurements. Our goal here is to describe the full range of natural histories exhibited by these taxa and to identify questions that require further study.
Over 70% of species in the large butterfly family Lycaenidae appear to be ant-associated, making them the largest single group of lepidopteran myrmecophiles (Tables 1 and 2). Two additional radiations of ant associates make up 20% of species in the closely related butterfly family Riodinidae (Table 2). While caterpillars in these two families are generally characterized as ant mutualists, we discuss evidence suggesting that interactions with negative consequences for ants are far more common than previously recognized, and that despite appearances, these associations might be better characterized as parasitic on the part of the lycaenids or, at best, reciprocally parasitic by both parties. Most other ant-associated groups, like the Australian moth family Cyclotornidae, are individually species-poor and rarely encountered but collectively span almost the entire lepidopteran tree of life and display great diversity, particularly in the tropics (Table 1). We show that myrmecophilous caterpillars that passively coexist with ants are far more diverse than previously recognized and suggest that many such caterpillar groups remain undiscovered.
Terminology and Overview
Myrmecophiles are “ant loving” organisms with adaptations that enable them to benefit from ant association, and we will refer to them interchangeably as ant associates (narrower definitions are also sometimes used: (Hölldobler and Wilson 1990; Kronauer and Pierce 2011; Nichols 1989). Specializations that help these species find or attract and subsequently stay in contact with ants are important and could be considered part of a basic signature of myrmecophily. Ants themselves, their pheromones, and even volatiles released by other organisms disturbed by ants are used as cues by adults or larvae to find ants, as discussed below. Within Lepidoptera, we consider caterpillars ant-associated if we can directly observe or infer from available evidence that caterpillars or ovipositing females use these cues to locate ants or that caterpillars themselves produce secretions or vibratory signals specialized to attract ants. Caterpillars may also qualify as ant-associated if they appear specialized to live in close proximity to ants on myrmecophytes, plants with a strong mutualistic relationship with ants and that typically provide ants with cavities for shelter.
Obligate ant associates are species that cannot complete their life cycle without ants. In cases where full life histories have been well documented, these species are easily identified. However, for cases where relationships must be inferred, a species is likely to be an obligate ant associate if the caterpillars are never found without ants nearby; if caterpillars rely on ants as a food source; if females hesitate or refuse to oviposit, even in captivity, without ants present; or if adults are typically only observed near the openings of ant nests. In contrast, facultative ant associates are sometimes found without ants. Facultative association of caterpillars with ants has only been well documented in Lycaenidae and Riodinidae, although it seems likely to occur in other groups that have not been so well characterized. Obligate ant associates usually associate with ants from only one genus or species, while most facultative myrmecophiles associate with multiple ant genera and subfamilies. A number of exceptions exist to these broad generalizations (Eastwood and Fraser 1999; Fiedler 2001; Glasier et al. 2018). For example, the obligately ant-associated Australian lycaenid, Jalmenus eichhorni , is attended by ants from different genera during the day and night (Dunn 2007). Larvae of a congener, J. evagoras , are typically associated with only a few ant species in the genus Iridomyrmex but during “breakout” periods of high abundance can readily be found associating with other genera (Pierce and Nash 1999).
Like other conditional interactions with ants, caterpillar-ant associations vary spatially and temporally, ranging from mutualisms , where both parties derive net fitness benefits from their interaction, through to parasitisms , where one party (in this case usually the ants) pays a fitness cost due to the association. Many appear to be commensal or only mildly parasitic in the sense that caterpillars benefit while ant fitness seems largely unaffected.
Many insects produce secretions that serve as a food source to attract and maintain a standing guard of ants and are described as being trophobiotic . We refer to lycaenid and riodinid caterpillars that do this as ant-attended . We use the term non-trophobiotic to describe caterpillars that are not actively ant-attended. The term “myrmecoxenous” has been used as a substitute for “non-trophobiotic” in recent literature but confusingly describes either a symphile, an insect that is a guest in ant nests (Nichols 1989), or a non-myrmecophile, an insect that is simply not ant-associated (Kitching and Luke 1985; Paul 1977), so we have avoided using it here.
Parasites found in ant nests often belong to groups that prey on ant-attended hemipterans and thus already possess appropriate defensive and feeding-related adaptations to coexist with ants (Eisner et al. 1972; Malicky 1970; Pierce 1995). These include numerous genera within the subfamily Miletinae [Lycaenidae], Shirozua [Lycaenidae], a few riodinids, Eublemma [Erebidae], Cyclotornidae, and perhaps Stathmopoda [Tineidae] and Baratrachedra [Coleophoridae]. This pattern is not confined to Lepidoptera: ant brood and trophallaxis feeding have been reported in species from nearly every prominent hemipteran-associated arthropod group, including ladybug beetles (Orivel et al. 2004; Vantaux et al. 2010), flower flies (Hölldobler and Wilson 1990), green lacewings (Tauber and Winterton 2014; Tauber et al. 2020), and even certain aphids themselves (Salazar et al. 2015).
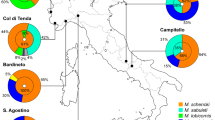
Many butterfly and moth larvae have ant associations that have been potentially overlooked because the relationship is defined largely by its absence: these are cases where ants cannot detect or appear indifferent to the caterpillars. These caterpillars typically only associate with ants near nests and food sources—habitats that are hotspots for lepidopteran ant associates more generally. For example, a veritable menagerie of potentially ant-associated Lepidoptera lives on the African ant-acacia Vachellia drepanolobium , the dominant tree species in the “black cotton” vertisols of East African savannas. Eighteen species of Lycaenidae, some attended by ants, were documented on these ant-plants at field sites in Kenya and Tanzania over a 5-year period (Fig. 1) (Baker et al. 2016; Martins et al. 2013; Whitaker et al. 2019). Numerous species of Tineidae, Tortricidae, Sesiidae, Blastobasidae, Gelechiidae, and Geometridae have been reared from the swollen thorn ant domatia of V. drepanolobium , and many others feed in the tree canopy (Adamski 2017; Agassiz 2011; Agassiz and Bidzilya 2016; Agassiz and Harper 2009; Agassiz and Kallies 2018; Baker et al. 2016; Hocking 1970). Some of these species are polyphagous and have been described as having greater abundance in the absence of ants (Agassiz 2011), and we would not describe these ones as being ant-associated. The majority are not sufficiently well known to be able to characterize them as ant-associated or not.
Lycaenid larvae, almost certainly Kipepeo kedonga (formerly known as Chilades kedonga (Parmentier et al. 2014)) that were abundant in swollen thorns of Vachellia drepanolobium in Suyian, Kenya. (Photo by Dino Martins)
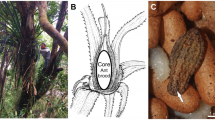
A few specialist myrmecophiles have nonetheless been documented on ant-plants. For example, larvae of Hystrichophora (Tortricidae) build strong, membranous, dome-like shelters within hollowed-out V. drepanolobium domatia that are frequently shared with ants (Fig. 2) (Agassiz 2011). Caterpillars of H. griseana are common on trees inhabited by colonies of Crematogaster mimosae or C. nigriceps , but they are almost never found on trees inhabited by colonies of Tetraponera penzigi (Baker et al. 2016). Similarly, caterpillars of Syssphinx mexicana (Saturniidae), Rosema dentifera (Notodontidae), and Coxina spp. (Erebidae) specialize on Central American acacias, Vachellia cornigera , and its relatives, which are inhabited by aggressive Pseudomyrmex ants, whose defenses the caterpillars are able to overcome (Janzen 1967; Janzen 1984; Janzen and Hallwachs 2021). The larvae of Dyops spp. (Noctuidae) are essentially immune to ant attack and feed on various species of Urticaceae, including Cecropia ant-plants defended by Azteca ants (Janzen and Hallwachs 2021; Ramos et al. 2018). Many other species reported from ant-plants may prove to be ant-associated upon further investigation. Tunnels and silk shelters built by Stenoma charitarca (Oecophoridae), and leaf rolls built by Acrospila gastralis (Crambidae), allow caterpillars to persist on Maieta guianensis plants occupied by Pheidole ants (Vasconcelos 1991), much as certain crambid larvae are protected from ants within leaf rolls on Tococa ant-plants (Michelangeli 2003). The database of macrocaterpillar food plants of the Area de Conservacion Guanacaste, Costa Rica (Janzen and Hallwachs 2021), does not indicate whether caterpillar host plants were actually occupied by ants but nonetheless includes dozens of butterfly and moth species that have been exclusively reared from ant-plant species, such as Lygropia cernalis (Crambidae) from Triplaris melaenodendron , Conchylodes nolckenialis (Crambidae) and Munona robpuschendorfi (Erebidae) from Cordia alliodora , and Macalla sp. (Pyralidae) from Cecropia obtusifolia . Many Lycaenidae and Riodinidae also prominently infiltrate ant-plants (e.g., DeVries and Baker 1989; Eastwood and Fraser 1999; Heredia and Robbins 2016; Heredia and Robbins 2016; Kaminski 2008b; Kaminski et al. 2010a; Kaminski et al. 2012b; Kaminski et al. 2020a; Maschwitz et al. 1984; Sands 1986; Shimizu-Kaya et al. 2015).
Many caterpillar species that do not directly interact with ants are polyphagous and occur on different host plants only as they become occupied by ants. For example, the obligate ant associations of many species in the butterfly tribe Liptenini (Lycaenidae) only became evident based on the observation that the large, attractive adults had only been observed around arboreal Crematogaster nests (see discussion below). Similarly, Homodes (Erebidae) are large and unusual caterpillars that occur on a wide variety of host plants but generally only when the plants are also patrolled by Oecophylla ants (see discussion below) (Fiedler 1991; Holloway 2005; Leong and D’Rozario 2012; Lokkers 1990). This kind of “cryptic” association probably exists even in less charismatic lepidopterans, such as leaf mining micromoths (compare Bily et al. (2008)).
Dejean et al. (2017) undertook the most extensive study to date of the extent of ant-caterpillar associations in tropical habitats. Defoliator and nectarivorous caterpillars were collected and reared from 50 to 100 m transects of the extrafloral nectary-bearing plant Alchornea cordifolia along forest edges in Cameroon, each transect exclusively dominated by one of five species of aggressive ants. Each of the tree-nesting species Crematogaster striatula , Oecophylla longinoda , Tetramorium aculeatum , and Camponotus brutus were represented by 30 transects, along with 10 transects dominated by the ground-nesting species Myrmicaria opaciventris . Of the 22 species of caterpillar found, only 1 was found with more than 1 ant species, although many were collected from numerous transects. All species showed distinct specializations to coexist with ants, including some parasites that could solicit trophallaxis or appeared to feed within ant nests. This study may be the first to systematically document the full spectrum of defoliator and nectarivorous caterpillars on a host plant dominated by specific ant species and shows that previously unknown ant associations across diverse lepidopteran families can be uncovered by careful observations in tropical habitats.
Synopsis of Caterpillar-Ant Associations
Tineidae and Psychidae
Diverse species of Tineidae and Psychidae are known to scavenge exclusively within ant nests, encased with debris or protected by silk webbing, and some of these probably feed on ant brood or food resources. Pending genus-level phylogenies that may reveal additional origins, ant associations appear to have originated independently in at least three tineid clades, represented respectively by the genera Myrmecozela , Setomorpha , and Amydria , as well as in the psychid genera Iphierga and Ardiosteres; see Regier et al. (2015) for a higher level molecular phylogeny of 62 representatives of the main lineages within Tineoidea) (Ahn et al. 2014; Gray 1974; Hinton 1951; Kistner 1982; Parmentier et al. 2014; Robinson and Nielsen 1993; Sanchez-Pena et al. 1993). Caterpillars in the Palearctic and Oriental genus Ippa (Tineidae) have been found in ant nests of Crematogaster (Myrmicinae), Polyrhachis , Lasius , Dolichoderus , and Anoplolepis (Formicinae) (Hinton 1951; Hölldobler and Kwapich in review). Ippa caterpillars build a flattened protective case, and while I. dolichoderella larvae in Java are only known to consume brood, I. conspersa larvae in Japan also feed on adult ants (Hinton 1951; Hölldobler and Kwapich in review). Although not obligately ant-associated, the free-living larvae of Perisceptis carnivora (Psychidae) in Panama build portable defensive cases and frequently feed on worker ants (Davis et al. 2008).
Tortricidae
Malaysian caterpillars of Semutophila saccharopa (Tortricidae) live in silk shelters constructed on bamboo and associate with ants from at least seven genera in a manner similar to aphids. Ants feed on the sugar-rich anal droplets provided by the caterpillars. The caterpillars prefer to excrete waste in the presence of ants, but the droplets can be withdrawn back into the anus and jettisoned several centimeters away from the larval shelter if ants remain unavailable (Maschwitz et al. 1986).
Cyclotornidae
In the Australian family Cyclotornidae, which comprises the single genus Cyclotorna, larvae start out as external parasites of ant-attended leafhoppers or scale insects (Fig. 3) (Dodd 1902, 1912; Pierce 1995). Second-instar larvae of Cyclotorna monocentra are flattened and produce an anal secretion that attracts ants. Workers of Iridomyrmex purpureus carry them into the nest, where they feed on brood until leaving to pupate under bark (Epstein et al. 1999; Pierce 1995). The Cyclotorna larvae will die if their anal secretions are not removed by ants (Hinton 1951). Epipyropidae, the apparent sister group to Cyclotornidae (Hall et al. 2004; Heikkila et al. 2015), are ectoparasites of planthoppers and cicadas (Hemiptera) but are not known to interact with ants (Pierce 1995).
Coleophoridae and Oecophoridae
Many Batrachedra (Coleophoridae) prey on scale insects, but larvae of the Indonesian species B. myrmecophila feed on ant brood in nests of Polyrhachis dives , protected from ants by portable cases (Hinton 1951; Pierce 1995). While several Stathmopoda spp. (Oecophoridae) feed on scale insects (Pierce 1995), one Australian species builds webs in Oecophylla nests where it may feed on ants (Downes and Edwards 2016).
Pyralidae
Many Pyralidae are associated with ants. Larvae of the Brazilian Pachypodistes goeldii (Chrysauginae) chew Dolichoderus gibbosoanalis nest cartons, which they use to construct a protective case, and may also feed on the brood (Hinton 1951; Pierce 1995). Adults of this species are covered in long, loose setae that are likely to help freshly eclosed adults escape attack by ants (Kistner 1982). An Australian species, Stenachroia myrmecophila (Galleriinae), may feed on Crematogaster brood (Hinton 1951; Pierce 1995). Larvae of other unidentified pyralids have been found in Dinomyrmex nest debris in Borneo (Orr et al. 1996) and in Oecophylla nests in Cameroon (Dejean et al. 2017). Caterpillar silk weaving may also help herbivorous Pyralidae coexist with ants. Dejean et al. (2017) found an unidentified species of pyralid that uses silk to cordon off young leaves of Alchornea cordifolia inhabited by Crematogaster striatula. Caterpillars of another unidentified pyralid species were found only on A. cordifolia occupied by Oecophylla longinoda , in communal caterpillar nests resembling Oecophylla nests from which they emerge at night to feed when the ants are less active. Crematogaster ants were recently found nesting within a shelter built by larvae of Triphassa (Pyralinae) on an Erica imbricata heath in South Africa [iNaturalist #23039584]. More work will be needed to determine if this remarkable relationship is coincidental or occurs regularly.
Other than Lycaenidae and Riodinidae, species of Tineidae and Pyralidae are the most prominent caterpillar guests in ant nests (Table 1). These species are herbivores, detritivores, and parasites and include the only caterpillars found in colonies of ants, such as leaf-cutter ants [Attini], that do not harvest nectar from plants and hemipterans (Kistner 1982; Robinson and Nielsen 1993; Sanchez-Pena et al. 2003). Other species of Tineidae and Pyralidae feed within social wasp, bee, termite, and even communal spider nests (Ahn et al. 2014; Brandl et al. 1996; Davis and Davis 2007; Deyrup et al. 2004; Kistner 1982; Pierce 1995). Most Lepidoptera found within human dwellings also belong to these two families (Bertone et al. 2016; Linsley 1944). Flexible diets, along with defenses that help larvae avoid aggression, may be among the factors that help these families to thrive alongside diverse host ant associates, and more species will undoubtedly be found in association with ants as new life histories are uncovered.
Crambidae
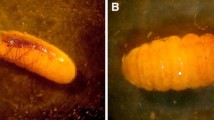
In the family Crambidae, at least two lineages in the largely phytophagous subfamily Spilomelinae may be associated with ants. Cirrhochrista saltusalis (Spilomelinae: Margaroniini) caterpillars have been found alongside Pheidole ants and Oboronia punctatus caterpillars (Lycaenidae) within debris nests constructed by the ants on flowerheads, but this cohabitation may be an unusual occurrence (Lamborn 1911; Lamborn et al. 1914). Immature stages remain unknown from most Wurthiini, but several feed on brood of arboreal ants, in addition to a single phytophagous species (Mally et al. 2019). Niphopyralis aurivillii (Spilomelinae: Wurthiini), a possibly chemical mimic of host ants known from Java, feeds on the brood of Polyrhachis bicolor and may help maintain the silken nest structure (Hinton 1951; Pierce 1995). Another species found in Java, N. myrmecophila , feeds on Oecophylla smaragdina brood and has a flattened portable case for protection (Hinton 1951). Niphopyralis chionesis is suspected to prey on brood of Oecophylla smaragdina in Australia (Pierce 1995), and Dejean et al. ( 2017) found a related larva feeding on Oecophylla longinoda eggs in Cameroon (Fig. 4).
(a, b) Caterpillar on an Oecophylla nest in Guinea, near Conakry. Larvae of this undescribed species near Niphopyralis (Crambidae) feed voraciously on weaver ant eggs (Dejean et al. 2017). (Photo by Piotr Naskrecki)
Erebidae
Larvae of lichen moths (Erebidae: Lithosiini) secrete toxins that protect them from ants (Chialvo et al. 2018; Palting 2020). Ayre (1958) observed hundreds of British Columbian Crambidia casta larvae that sheltered and pupated in Formica nests, although this behavior has not been found in other populations of this species (Palting 2020). Larvae of another small lichen moth found in Japan, Nudina artaxidia, are obligate associates of Lasius ants and feed on honeydew from scale insects, along with lichen (chapter frontispiece) (Komatsu and Itino 2014).
Many Eublemma spp . (Erebidae: Boletobiinae) feed on scale insects, where they are concealed from attending ants by a portable protective casing (Dejean et al. 2016; Lamborn et al. 1914; Pierce 1995; Susilo and Susilo 2015). In Cameroon, Dejean et al. (2016) found that Eublemma albifascia lays eggs on ant nests, and first-instar caterpillars are carried into Oecophylla longinoda brood chambers by workers. Subsequent instars are fed by ants and steal from trophallaxis between workers, and ants groom their bodies and drink their anal secretions. The larvae acquire colony odors and do not require physical protection from host ants (Dejean et al. 2016). Dejean et al. (2017) found 359 caterpillars of Eublemma albifascia in only four colonies of Oecophylla longinoda. Due to their intense trophallaxis requirements, Eublemma albifascia parasites generally cause the death of the queen through neglect, though their numbers are regulated by some parasitoid wasps (Dejean et al. 2016). Eclosed adults are mostly ignored and, if occasionally attacked, are protected by long, dense scales (Dejean et al. 2016).
In a few wasmannian ant mimics, the same specialized tactile structures are used to integrate with ants and to scare off other predators (von Beeren et al. 2018; Kronauer and Pierce 2011). A few Oriental and Australasian species of the genus Homodes (Erebidae: Boletobiinae) occur on a wide range of host plants but never far from Oecophylla smaragdina weaver ants (Fiedler 1991; Holloway 2005; Leong and D’Rozario 2012, iNaturalist #65316827, iNaturalist #27728866). These caterpillars are excellent mimics of Oecophylla ants at both the front and the back, with a false head on the posterior abdomen and long clubbed setae resembling ant appendages (Fig. 5). Waving these setae not only deters visual predators but appears to placate Oecophylla workers (video at https://www.facebook.com/watch/?v=1938845709677099) (Entomological Network of Singapore 2017). Structurally similar, possibly glandular setae are found on the thorax and abdomen of related larvae documented on iNaturalist, which are not known to be ant-associated [e.g., iNaturalist #21087410, iNaturalist #38085822, iNaturalist #21414510]. Lokkers (1990) found ant-mimicking looper moth caterpillars in north Queensland exclusively on Oecophylla-occupied trees, which may have been larvae of Homodes or another group with a similar life history.
Notodontidae
Phytophagous larvae of Stauropus and Neostauropus (Notodontidae) have enlarged mesothoracic and metathoracic legs used to mimic ants in early instars, and spiders once larvae become larger, with a terrifying threat display (Fig. 6) (Poulton 1890; Pratt et al. 2016). In Britain, photographer Andy Newman experimentally brought together first-instar Stauropus fagi larvae and Formica ants and discovered that larvae were ignored after waving their mesothoracic legs and contacting the ants’ antennae [http://www.andynewman.org/html/lobster_moth.html]. Dejean et al. (2017) discovered related larvae in Cameroon that use their enlarged mesothoracic legs to solicit trophallaxis from associated Oecophylla longinoda ants. The larvae also fed on young leaves and extrafloral nectaries. An unidentified larva of this species from southern Nigeria may have also been described by Farquharson et al. (1922). Larvae of Afrotropical Amyops ingens strongly resemble Stauropus larvae and have much shorter, but still notably elongated, thoracic legs of unknown function [(iNaturalist #11244196, iNaturalist #11446507]). Perhaps they are used to handle soft-bodied Hemiptera or honeydew as in some Lycaenidae and Riodinidae (DeVries and Penz 2000; Dejean et al. 2017). The biology of these fascinating Notodontidae remains largely undocumented; more research is needed to understand their ecology and diversity.
Papilionoidea (Hesperiidae, Nymphalidae, Pieridae)
With over 900 well-documented and more than 4000 inferred myrmecophilous species, the butterfly families Lycaenidae and Riodinidae account for an overwhelming proportion of caterpillar-ant associations (Table 2). At least a few butterfly species in other families are also ant-associated. Malaysian Lotongus calathus caterpillars (Hesperiidae) build leaf shelters that are always shared with nesting Dolichoderus ants (Igarashi and Fukuda 1997). Chemically protected larvae of Neotropical Vettius tertianus (Hesperiidae) are usually found living with predatory ants in ant gardens, although not enough is known of their biology to conclude whether or not they are true myrmecophiles (Orivel and Dejean 2000).
Ants gathering to drink from leaf exudates generated by herbivores are not uncommon, although rarely analyzed, and result in facultative ant interaction with caterpillars of various butterfly and moth species (Fiedler 1991; Larsen 2005). For example, Young (1978) observed ants using their antennae to stroke a larva of the nymphalid butterfly Mechanitis isthmia in Costa Rica, whereupon the larva would withdraw from the leaf edge and allow the ants to drink exudates from the newly cut surface. Diverse ants commonly drink from the feeding sites of Catopsilia larvae (Pieridae), and some ant species appear to find the caterpillars themselves more attractive than the leaf exudates (Williams 1995-2020, iNaturalist #10726006 iNaturalist #15027508, http://pureoxygengenerators.blogspot.com/2017/10/some-nature-finds.html, https://www.flickr.com/photos/129254524@N06/16162943814/). Larvae of many Pieridae and Saturniidae produce potent secretions to deter ants, and occasional reports suggest that the secretions themselves are consumed by ants under rare circumstances (Fig. 7) (Fiedler 1991; Hinton 1951; Smedley et al. 2002).
Glistening droplets on spines of larva of Phoebis philea (Pieridae) feeding on Senna mexicana being inspected by an unidentified ant, with a second ant feeding on an extrafloral nectary nearby. The droplets are thought to be defensive but may in some cases (depending on the ant species, host plant, and location) be strikingly attractive to ants (e.g., photo of Catopsilia pyranthe surrounded by Anoplolepis gracilipes ants at http://pureoxygengenerators.blogspot.com/2017/10/some-nature-finds.html). (Photo by James Spencer, kindly provided by Nadia Spencer)
Ant Association in the Lycaenidae and Riodinidae
Throughout Lepidoptera, only the families Lycaenidae and Riodinidae contain ant-associated taxa that number more than a few dozen species. The ability to actively attract ants with food rewards and sophisticated signaling may help account for their surprisingly massive radiation compared with other ant-associated larvae whose interactions are more limited and rarely involve food rewards. Non-trophobiotic myrmecophiles are limited to ant “hotspots,” where enemy-free space is strongest and unique resources are available: either around ant-attended hemipterans, within ant nests, on ant-plants, or within the arboreal territories of highly aggressive ants like Oecophylla . Correspondingly, trophobiotic organs in Lycaenidae and Riodinidae that obligately occur around ant-tended hemipterans and ant nests are often lost or modified, most notably in the lycaenid subfamilies Miletinae and Poritiinae and in riodinids like Aricoris arenarum (Kaminski et al. 2020b; Shimizu-kaya et al. 2013).
Recent comparative analyses using a well-resolved tribal level phylogeny of butterflies indicate that ant association arose once in the ancestor of the Lycaenidae nearly 80 mya, twice more recently in its sister family, the Riodinidae, once in the subtribe Eurybiina, and once in the Nymphidiini (Espeland et al. 2018). Thus, similar traits used in ant-caterpillar associations appear to have arisen independently at least three times in these two butterfly families.
Adaptations of Adults
Ant-related visual and chemical cues are used during mate finding and oviposition by many ant-associated Lycaenidae and Riodinidae (e.g., Atsatt 1981b; Casacci et al. 2019b; Dejean et al. 2017; DeVries 1997; Elgar and Pierce 1988; Elgar et al. 2016; Fiedler and Maschwitz 1989a; Fiedler and Maschwitz 1989b; Fraser et al. 2002; Kaminski et al. 2013; Heath 1997; Henning 1983; Kaminski and Carvalho-Filho 2012; Martins et al. 2013; Pierce 1984; Pierce and Elgar 1985; Pierce and Nash 1999; van der Poorten and van der Poorten 2016; Pringle et al. 1994; Seufert and Fiedler 1996; Williams 1995-2020), even in species that are facultatively ant-attended (Mota and Oliveira 2016; Wagner and Kurina 1997) or non-trophobiotic (Bächtold et al. 2014; Fiedler and Maschwitz 1989b; Funk 1975; Sáfián and Collins 2014; Sáfián and Larsen 2009; Rodrigues et al. 2010). Many obligate ant associates will not oviposit unless ants are present (e.g., Heath 1997)). Chemical eavesdropping on ants is widespread among myrmecophiles, and lycaenid adults may detect ant pheromones as well as visual cues (e.g., Adams et al. 2020; Kaliszewska et al. 2015; Sáfián and Larsen 2009; Williams 1995-2020). Visual and chemical cues are also used by non-myrmecophiles to avoid ovipositing near ant territories (Freitas and Oliveira 1996; Van Mele et al. 2009; Sendoya et al. 2009).
Phengaris (=Maculinea ) is one of two lycaenid genera with species whose larvae are obligately phyto-predaceous, with eggs laid on specific plant hosts that serve as food for the early instars and that later drop to the ground to be carried by workers into the ant nest, where they feed on the brood or solicit regurgitations to complete development. Recent research on ovipositing females of Phengaris species has started to resolve a longstanding puzzle regarding whether or not these parasitic butterflies use ants as cues to locate oviposition sites (Carleial et al. 2018; Casacci et al. 2019b; Czekes et al. 2014; van Dyck and Regniers 2010; Fürst and Nash 2010; Musche et al. 2006; Patricelli et al. 2011; Thomas and Elmes 2001; Wynhoff et al. 2008; Wynhoff et al. 2015). Apparently Myrmica ants nesting at the base of Origanum vulgare plants (Lamiaceae) damage the roots and thereby induce the plants to release defense-related volatile organic compounds, or VOCs, including the monoterpenoid carvacrol and its isomer thymol. Ovipositing females of Phengaris arion can detect these compounds and use them to identify plants with appropriate ant hosts located beneath them (Pech et al. 2007; Patricelli etal 2015). The larvae of other species of Phengaris also feed on host plants in the Gentianaceae and Rosaceae (Als et al. 2004), and it seems likely that a similar mechanism exists on other host plants whereby damage to plant roots caused by ant colonies nesting underground may induce the release of VOCs that attract ovipositing females. Cues from a number of different plant families may be used by ovipositing females in this way, but this remains to be tested.
Chemical signals seem to mediate ant interactions with adults of many lycaenid and riodinid butterflies, generally with ants that are also associated with caterpillars (Atsatt 1981a; Farquharson et al. 1922; Fiedler and Maschwitz 1989a; Pierce et al. 2002). These semiochemicals may be particularly important in species that pupate within ant nests (Elfferich 1998; Lohman 2004). Various adult Lycaenidae and Riodinidae are inspected or groomed by ants (DeVries 1984; Fiedler and Maschwitz 1989b; van der Poorten and van der Poorten 2016, iNaturalist #36616206, iNaturalist #5526494, iNaturalist #62627204, iNaturalist #56774612, iNaturalist #66838365). Adults of most Poritiinae and Miletinae (Lycaenidae) feed exclusively from extrafloral nectaries and carbohydrate-rich insect exudates, both frequently attended by workers of the same ant species that are associated with their own larvae (Figs. 8 and 9) (Atsatt 1981a; Callaghan 1992b; Cottrell 1984; Dejean et al. 2017; Farquharson et al. 1922; Fiedler and Maschwitz 1989b). Certain Riodinidae may have similar habits (Torres and Pomerantz 2016).
Adaptations of Caterpillars and Pupae
Before pupation, and in some species whenever not feeding, larvae of diverse Riodinidae (e.g., DeVries 1997; Kaminski and Carvalho-Filho 2012; Kaminski et al. 2020b; Ross 1966) and Lycaenidae enter special shelters built for them by ants (e.g., Eastwood et al. 2005; Eastwood et al. 2008a; Ekka and Rastogi 2019; Webster and Nielsen 1984) or the ants’ nests themselves (e.g., Benyamini and Bálint 1995; Bury and Savchuk 2015; Mizuno et al. 2019; Wagner 1995). These cohabitation behaviors appear to co-opt existing ant behaviors widely used to shelter hemipterans. Many caterpillars in seasonally arid and cold regions enter underground ant nests, likely to escape unfavorable conditions. The need to escape the increasingly dry conditions and the associated risk of fires that occurred during the aridification of Africa in the Miocene may have been an important driver leading to the relatively large number of obligately parasitic relationships found in the dry savanna habitats of southern Africa and Australia. These regions are also hotspots for myrmecochorous plants, those plants with seeds dispersed by ants (Lengyel et al. 2010), possibly for similar reasons, although the phosphorus-poor soils of these regions are also likely to have been important (see discussion below) (Westoby et al. 1982). Larvae of a number of species have been reported to follow ant trail pheromones, but only a few cases of this behavior have been experimentally confirmed (Dejean and Beugnon 1996; Fiedler et al. 1996).
Hinton (1951) noted that ant-attended larvae, even within ant nests, may be attacked if ants are sufficiently alarmed by an intruder. Most lycaenid larvae can retract their head beneath a sclerotized prothoracic plate and are ventrally flattened, shielding vulnerable body parts (Ballmer and Pratt 1988; Fiedler 1991; Malicky 1969; Malicky 1970; Pierce et al. 2002). Larvae that live in close proximity with ants may have a wrinkled cuticle up to 20 times thicker than that of other Lepidoptera to avoid harm from the occasional bite (Bächtold and Alves-Silva 2013; Fiedler 1991; Gnatzy et al. 2017; Malicky 1969; Malicky 1970). In general, those with facultative associations with ants have thicker cuticles than those with obligate associations, although this depends in part on the mandible size of the ant associates (Dupont 2012). Lycaenid caterpillars also generally lack the thrash reflex to disturbance found in other Lepidoptera, which can elicit enhanced attack from ants (Bächtold and Alves-Silva 2013; Fiedler 1991).
Ant-attended Lycaenidae and Riodinidae possess a variety of multimodal “ant organs” to attract and signal to ants via chemicals or stridulation. Cuticular hydrocarbons and similar substances protect lycaenid larvae from most ant aggression, as described in a later section. In addition, many ant-associated lycaenid and riodinid caterpillars are attractive to ants, which groom and antennate various parts of their bodies. Ants are often drawn to specific parts of lycaenid larvae bearing dense single-celled epidermal glands that Malicky (Malicky 1970) described in English as “perforated cupola organs” (PCOs). Kitching (g 1983) translated Malicky’s original “porenkuppeln” (Malicky 1969) as “pore cupola organs” (PCOs), and this term has been adopted generally. PCOs are also found in many pupae (e.g., Duarte et al. 2001; Fiedler 1989b; Fiedler and Seufert 1995; Hinton 1951; Malicky 1970; Pierce and Nash 1999). PCOs or putative homologs have been found in the larvae of all Lycaenidae and Riodinidae that have been examined (Dupont et al. 2016; Fiedler 1991; Mota et al. 2014; Nielsen and Kaminski 2018; Pierce et al. 2002; Santos et al. 2014). As a result, Pierce et al. (2002) suggested that PCOs may represent a key preadaptation for the radiations of myrmecophilous Lycaenidae and Riodinidae. The ant-associated functions of these organs are likely to be convergent given what we now know about the phylogeny of these groups. The function of PCOs in non-myrmecophilous caterpillars has not been carefully explored: PCOs are widespread among caterpillars of non-myrmecophilous Riodinidae as well as the non-myrmecophilous family Hesperiidae, where they were originally called “lenticles” (DeVries 1991c; Franzl et al. 1984).
Larval PCOs are often concentrated around spiracles and secretory organs (e.g., Downey and Allyn 1979; Fiedler 1991; Kitching and Luke 1985; Mota et al. 2014; Mota et al. 2020; Pierce and Nash 1999). Many Lycaenidae also have a higher density of PCOs on thoracic segments that are attractive to ants (Pierce and Nash 1999). Comparing related species or different populations of a single species, PCOs may be more numerous or productive in larvae that are more closely ant-associated (e.g., Ballmer and Pratt 1991; Kaminski et al. 2013).
In addition, a large number of wedge-shaped, dendritic, mushroom, and other highly modified setae appear important to ant interactions of various larvae and pupae (DeVries et al. 1986; Downey and Allyn 1979; Duarte et al. 2001; Dupont et al. 2016; Fiedler 1989a; Fiedler 1991; Hall and Harvey 2001; Hall et al. 2004; Kaminski and Carvalho-Filho 2012; Kaminski et al. 2013; Kaminski et al. 2020b; Pierce et al. 2002). The presence of dendritic setae appears to be strongly correlated with the ants’ interest in larvae (Ballmer and Pratt 1991). These specialized setae are generally concentrated near PCOs and other secretory organs and may help disperse secretions to arouse ants (Ballmer and Pratt 1991). Others are mechanoreceptors that respond to attending ants (Tautz and Fiedler 1992).
Tentacle organs (TOs) are paired, typically eversible structures on the eighth abdominal segment of many riodinid and lycaenid larvae that are operated hydrostatically by specialized muscles (Fig. 10) (Basu and Kunte 2020; Gnatzy et al. 2017; Hinton 1951; Vegliante and Hasenfuss 2012). While TOs are potentially part of the lycaenid and riodinid ground-plan, they are absent in the riodinid subfamily Nemeobiinae, the lycaenid subfamilies Poritiinae and Lycaeninae, all of the Miletinae except the genus Aslauga, and a few other genera (Campbell and Pierce 2003; Fiedler 1991; Pierce et al. 2002). Their function is usually defensive and often specialized to signal to ants as discussed below.
Vibratory Signaling
Larvae of various Lepidoptera produce vibratory signals to deter predators, defend larval territories, or attract additional larvae (see Yack, Ch. 7) (e.g., Bura et al. 2009; Bura et al. 2011; Dookie et al. 2017; Fletcher et al. 2006; Sanetra and Fiedler 1996; Yack et al. 2001; Yadav et al. 2017). Stridulations are a widespread method for ants to recruit nestmates for foraging or defense and have correspondingly been adapted by some larvae to attract attention (Schönrogge et al. 2017). One of the earliest reports of larval stridulation came from naturalist Charles O. Farquharson, who noted a sensation like an electric shock from touching different lycaenid caterpillars (Farquharson et al. 1922). Substrate-borne acoustic signals produced by numerous lycaenid and riodinid larvae encourage ant attendance and are similar to those made by attending ants (e.g., Fiedler et al. 1996; Lin et al. 2019; Riva et al. 2017; Schurian and Fiedler 1994; Travassos and Pierce 2000). Larval sounds or sound-producing organs have been observed in all examined ant-attended lycaenid and riodinid larvae and are only known to be absent in some non-myrmecophilous Riodinidae and New World Lycaenidae of the tribe Eumaeini (DeVries 1990; DeVries 1991d). Some non-trophobiotic larvae are able to produce sounds, but all belong to genera that facultatively associate with ants (Elfferich 1998; Pierce et al. 2002; Riva et al. 2017).
The few described sound production mechanisms in lycaenid larvae are all stridulatory (Hill 1993; Schönrogge et al. 2017; Schurian and Fiedler 1994). The stridulatory organ of both the larva and pupa of Arhopala madytus is located between the fifth and sixth abdominal segments (Hill 1993), as is the stridulatory organ of most lycaenid pupae (Downey 1966). However, in the pupa, the file (sixth segment) is posterior to the stridulatory plate (fifth segment), whereas in the larva of A. madytus, their placements are reversed. The discrete organs giving rise to these substrate-borne vibrations have proved difficult to identify in many species. In the Australian lycaenid, Jalmenus evagoras , they seem likely to consist of rings of tiny, serially repeating teeth and scrapers occurring between each pair of larval abdominal segments. When the larva is calling, these areas can be seen to vibrate using high speed video (Pierce et al. 2002; Travassos and Pierce 2000).
Pupae of Lycaenidae and Riodinidae also produce several types of vibrations, including “chirping” noises audible to humans, using plate-and-file stridulatory mechanisms located on membranes between abdominal segments 4 and 7 (Downey and Allyn 1973; Downey and Allyn 1978). In addition, “tooth-cast” systems, in which one opposing structure of the sound-producing organ is an imprint of the other, are found in diverse Lycaenidae (Downey and Allyn 1973), as in pupae of Nymphalidae and Papilionidae (Dolle et al. 2018). Acoustic signals play an important role in ant recruitment and appeasement by myrmecophilous Lycaenidae and Riodinidae but are also widespread in non-myrmecophilous pupae, presumably serving as deimatic displays to startle predators as in other Lepidoptera (Dodd 1916; Dolle et al. 2018; Downey and Allyn 1973; Elfferich 1998; Lin et al. 2019; Pierce et al. 2002; Travassos and Pierce 2000).
Lycaenidae
The Lycaenidae contain over 5000 species in more than 400 genera distributed worldwide (Eliot 1973; Espeland et al. 2018; Pierce et al. 2002). Although different species vary in the relative strength and context of ant association, all lycaenid subfamilies have species that are either ant-attended or form some kind of regular association with ants (Table 2).
Curetinae
The lycaenid subfamily Curetinae consists of a single genus (Curetis ) of 18 species and is distributed from India to the Solomon Islands (Eliot 1990). The genus is significant inasmuch as it is sister to all other Lycaenidae and may illustrate plesiomorphic traits shared with riodinids but lost in other lycaenids (Espeland et al. 2018). Curetis larvae can produce loud substrate-borne vibrations (Fiedler et al. 1995). Curetis TOs are housed in large, sclerotized cylinders, which evert long filamentous processes when the larva is disturbed, exciting nearby ants (videos at https://www.youtube.com/watch?v=2AAg26XDtgM, https://www.youtube.com/watch?v=zhSX_7edW44) (DeVries 1984). Much like those of some non-trophobiotic riodinids described below (Nielsen and Kaminski 2018), Curetis TOs evert and appear to emit repulsive chemicals, in response to ants and other attackers including parasitoid flies and wasps (video at https://www.youtube.com/watch?v=LUKxmq3_6MU) (Ballmer 2015; DeVries et al. 1986; Fiedler et al. 1995; de Niceville 1890; van der Poorten and van der Poorten 2016). Ants usually show little interest in Curetis larvae but often accompany them to drink from leaf exudates where larvae have been feeding (Fig. 11) (DeVries 1984; Fiedler et al. 1995).
The remaining Lycaenidae form a clade that is ancestrally ant-attended (Espeland et al. 2018). Most species of the subfamilies Aphnaeinae, Theclinae, and Polyommatinae have a dorsal nectary organ [DNO], a unique slit-like glandular invagination on the 7th abdominal segment that produces attractive secretions for ants and appears in the 2nd or 3rd instar (Daniels et al. 2005; Fiedler 1991; Hinton 1951; Pierce et al. 2002). A superficially similar abdominal invagination found in Curetinae may be a vestigial DNO or perhaps simply a muscle attachment site (DeVries et al. 1986). The DNO contains 2–4 individual glands, which structurally and developmentally resemble modified setae (Hinton 1951; Malicky 1970; Newcomer 1912; Pierce and Nash 1999; Vegliante and Hasenfuss 2012). Muscles around the DNO usually allow it to push upward and extrude liquid droplets or retract and suck back these secretions (video at https://www.youtube.com/watch?v=fCho3Vrt2bU) (Basu and Kunte 2020; Pierce and Nash 1999). Larvae of many obligately ant-attended species have been reported to die in captivity from mold and/or infection without ants to remove built-up secretions around the opening of the DNO (Cottrell 1984; Hinton 1951; Williams 1995-2020).
Caterpillars of some species have been shown experimentally to deploy their DNO secretions strategically, increasing the rate of droplets provided when they are vulnerable or under perceived attack and decreasing per capita secretions in larger larval aggregations (Agrawal and Fordyce 2000; Axen and Pierce 1998; Axén et al. 1996; Leimar and Axén 1993). Caterpillars may also increase secretion rates when more ants are present; this might allow them to retain a larger retinue of ants (Axén 2000; Fiedler and Hagemann 1992). Curiously, the dorsal nectary organ remains functional in many parasites that enter the ant nest such as Niphanda fusca and species of Phengaris, suggesting that secretions from the DNO in these species may contain essential substances enabling them to manipulate attendant ants.
The TOs of species in the Aphnaeinae and the Theclinae-Polyommatinae assemblage appear to secrete volatile chemicals that excite ants to defend the larva (Casacci et al. 2019b; Fiedler 1991; Fiedler et al. 1996; Henning 1983; Pierce et al. 2002). Lycaenid TOs are most frequently everted to attract ants when caterpillars are disturbed or are traveling to a new location or when ant-caterpillar interactions first begin (Axén et al. 1996; Fiedler et al. 1996; Fiedler and Hagemann 1992; Leimar and Axén 1993). Secretions from the tentacle organs of lycaenids have been difficult to detect and/or characterize chemically (Gnatzy et al. 2017; Pierce and Nash 1999). The TOs of the Japanese species Shirozua jonasi (Theclinae: Theclini) were described to contain dendrolasin (Yamagushi and Shirozu 1988), a compound found in some ant alarm pheromones (Hölldobler and Wilson 1990). Although the chemicals involved are unknown, extracts from the TOs of Aleiodes dentatis (Aphnaeinae) were shown to elicit an alarm response from workers of the attendant ant species (Henning 1983). Alarm pheromones are also mimicked by many myrmecophilous rove beetles and wasps (Stoeffler et al. 2007; Thomas et al. 2002).
In terms of delivery, some authors have speculated that the tentacle organs of Lycaenidae might disperse chemical signals that are coated on their long, finely branched apical setae when the tentacle is withdrawn into an evagination formed by the cuticle (Fiedler et al. 1996; Fiedler et al. 1995; Hinton 1951; Kitching and Luke 1985; Pierce and Nash 1999; Sanetra and Fiedler 1996). Additional research is warranted, as Gnatzy et al. (2017) carefully examined the histology of these setae and found no evidence that they were glandular in nature.
The Theclinae-Polyommatinae Assemblage
Theclinae and Polyommatinae are both polyphyletic as traditionally defined but together form a well-supported monophyletic group (Espeland et al. 2018). The Theclinae-Polyommatinae assemblage is widespread, including over 4000 species in nearly 350 genera. Larvae are mostly phytophagous and ant-associated, but several lineages are non-myrmecophilous (Table 2, Fig. 12, videos at https://www.youtube.com/watch?v=GsSlcA0WXnk, https://www.youtube.com/watch?v=43vmltWoSdo].
With over 1300 species that typically only form facultative ant associations, the tribe Polyommatini is the largest tribe of Lycaenidae. Only a few obligately ant-associated taxa are known in this tribe outside of the two unique genera, Lepidochrysops and Phengaris (Polyommatini). Larvae of the some 130 species of Afrotropical Lepidochrysops typically feed on flowers until the 3rd instar, when they begin to mimic ant brood and are carried by workers of species of Camponotus (subfamily Formicinae) into the nest to feed on brood and/or engage in trophallaxis (Heath and Claassens 2003; Henning 1983).
Like Lepidochrysops , the approximately ten species of Palearctic Phengaris (= Maculinea ) are also phyto-predaceous. The larvae of different species of Phengaris initially feed on flowers and in the fourth instar are carried by Myrmica workers (subfamily Myrmicinae) into the nest, where different larvae, even those derived from eggs laid in the same year, will remain parasitic for either 1 or 2 years (Elmes et al. 2019; Thomas et al. 1998; Witek et al. 2006). Acceptance of Phengaris by host ants is mediated by specialized chemical mimicry of ant hosts (Akino et al. 1999; Casacci et al. 2019b; Casacci et al. 2019a; Nash et al. 2008; Schönrogge et al. 2004; Solazzo et al. 2013). Although most Phengaris feed directly on ant brood, a group of “cuckoo” species have larvae that specialize on trophallaxis (Als et al. 2004; Thomas and Elmes 1998). Both predatory Phengaris arion and cuckoo Phengaris rebeli are nest parasites whose larvae have been reported to produce acoustic signals resembling those of their host ant queens and giving them extreme priority in feeding and protection (Barbero et al. 2009a; Barbero et al. 2009b; Barbero et al. 2012; Sala et al. 2014; Thomas et al. 2013). Most Phengaris species can parasitize nests of multiple ant species, although local populations are often strongly specialized on different hosts (Pech et al. 2007; Tartally et al. 2019; Ueda et al. 2016; Witek et al. 2011; Witek et al. 2008; Sielezniew et al. 2010; Thomas et al. 2013). Phengaris arion has become a classic conservation success story, after recognition of its obligate relationship with a single ecologically restricted Myrmica species in the UK facilitated the reintroduction of the caterpillar species (Thomas et al. 2009).
First-instar larvae of East Asian Niphanda fusca (Niphandini) feed on aphid honeydew, but later-instar larvae enter Camponotus nests, where they chemically mimic male ants and are fed by workers (Hojo et al. 2014a; Hojo et al. 2009). Larvae of Phengaris , Lepidochrysops , and Niphanda fusca that enter the ant nest in later instars have an unusual growth pattern, growing more than ten times as much once in the ant nest as would be predicted from their earlier stages (Elmes et al. 2001). Two Afrotropical species of Anthene (Lycaenesthini) are parasites in nests of species of Crematogaster (Williams 1995-2020). A few related larvae—Tropical Asian Chilades lajus (Polyommatini) and Afrotropical Triclema lamias (Lycaenesthini)—may prey on aphids and scale insects (Farquharson et al. 1922; Pierce 1995). Many other plant-feeding species supplement their larval diet with hemipteran honeydew under certain conditions (Fig. 13) (e.g., Pierce and Elgar 1985).
Only a few other parasitic species can be found within the remaining tribes that are currently non-monophyletically grouped as Theclinae. All 11 species of the Australian genus Acrodipsas (Eastwood and Hughes 2003; Miller and Lane 2004; Sands and Sands 2015) and a few species within the mostly phytophagous and highly ant-associated genera Ogyris and Arhopala are brood predators in ant nests (Braby 2000; Fiedler 2012; Pierce 1995). Palearctic Shirozua larvae mostly feed on hemipterans and their excretions but also sometimes on Lasius or Camponotus ant trophallaxis (Fiedler 2012; Pierce 1995; Zhou and Zhuang 2018). Shirozua jonasi may enter ant nests to pupate, and adults are protected by dense cotton-like hairs (Cottrell 1984).
Although widely distributed, over 90% of the approximately 1096 species in the tribe Eumaeini are found in the Neotropical region, and all are either non-myrmecophilous or facultatively so, usually only sporadically ant-attended. The Old World taxa are clustered in a single clade consisting largely of the species-rich sections Callophrys , Erora , and Satyrium . Their huge radiation appears to be associated with intense sexual selection, as males have a great diversity of secondary sexual traits such as brush organs associated with the genitalia and androconial wing scent pads and patches that waft pheromones (Valencia-Montoya et al. In review). Caterpillars of several genera are aposematically colored or bear defensive tubercles and scoli, resembling Limacodidae (Fig. 14) (e.g., Kaminski et al. 2010b; Silva et al. 2014). Some respond to disturbance by curling their body or hanging off the substrate on a silk thread, behaviors otherwise unknown in Lycaenidae (Fiedler 1991; Silva et al. 2014). The approximately 175 species in the detritivorous Neotropical subtribe Calycopidina have never been reported with ants, but limited evidence suggest that some species might be facultatively ant-associated (Duarte and Robbins 2010; Nishida and Robbins 2020, supplemental table from Schär et al. 2018; Silva et al. 2014).
A number of studies have looked at the developmental effects of ant attendance on caterpillars of the Theclinae-Polyommatinae assemblage. Different attendant ant species differ in their impact on survival and development (Fraser et al. 2001; Kaminski and Rodrigues 2011; Mizuno et al. 2019; Trager and Daniels 2009; Saarinen and Daniels 2006; Wagner 1993). The costs and benefits of ant attendance are also borne differently by males and females, probably based on differing physiological demands on adults of each sex to ensure reproductive success (Mizuno et al. 2019; Pierce et al. 1987). Measured effects of ant attendance on developmental times and adult sizes vary extensively between different species (Baylis and Pierce 1992; Cushman et al. 1994; Fiedler and Hölldobler 1992; Fiedler and Hummel 1995; Fiedler and Saam 1994; Fraser et al. 2001; Kaminski and Rodrigues 2011; Mizuno et al. 2019; Pierce and Nash 1999; Pierce et al. 1987; Robbins 1991; Saarinen and Daniels 2006; Trager et al. 2013; Wagner 1993). The methods employed in quite a few of these studies involve placing ants and larvae together in disturbed laboratory environments in order to create an “ant-attended” treatment. Controlled experiments using intact ant colonies containing queens and with naturally foraging workers tending caterpillars feeding on live host plants are difficult to carry out, but they seem likely to yield different results from treatments in which individual workers are simply enclosed with caterpillars feeding on cuttings to simulate natural tending. For example, field versus laboratory experiments found different effects on developmental times of facultatively ant-associated larvae of Glaucopsyche lygdamus (Fraser et al. 2001; Pierce and Easteal 1986).
Leguminous host plant use is broadly correlated with ant attendance within the Theclinae-Polyommatinae assemblage (Fiedler 1995; Pellissier et al. 2012a; Pierce 1985). The relationship may not be causal, but protein-rich foods could help caterpillars produce nitrogen-rich secretions for ants. For example, individual larvae of Jalmenus evagoras larvae are tended by more ants per capita when they are fed higher-quality host plants that have been treated with nitrogenous fertilizer than when feeding on lower-quality control plants (Baylis and Pierce 1991). Similarly, feeding on flowers may lead to greater larval growth and in some cases has been shown to increase the volume of DNO secretions (Burghardt and Fiedler 1996; Collier 2007; Pierce and Easteal 1986; Wagner and Kurina 1997). The distribution of legumes and their symbiotic bacteria might also exert indirect effects on lycaenid biogeography (Steidinger et al. 2019). Feeding on Fabaceae appears to be an ancestral state of all phytophagous lycaenid subfamilies with the exception of the Lycaeninae (Boyle et al. 2015; Espeland et al. 2018; Fiedler 1991). Thus, the correlation between ant attendance and legume feeding might be more appropriately viewed as one where species that switch to less nutritious food sources are unlikely to remain ant-attended (Fiedler 1995).
Lycaeninae
The approximately 110 species of Lycaeninae, which form a sister group to the Theclinae-Polyommatinae assemblage, have an unusually wide, disjunct distribution that includes all major zoogeographic regions. All described species of Lycaeninae lack a dorsal nectary organ and tentacle organs, but larvae and pupae possess stridulatory organs and sometimes enter ant nests (Bascombe et al. 1999; DeVries 1991d; Downey and Allyn 1973; Fiedler 1991; Gibbs 1980; Heath and Claassens 2003; Yago et al. 2010). Furthermore, a few species have been reported possibly to rely on ants for oviposition, and these caterpillars may also be somewhat attractive to ants (Ballmer and Pratt 1991; Fiedler 1989a; Funk 1975; Oliver 2007).
Miletinae
The lycaenid subfamily Miletinae is notably missing from the Neotropics and western Palearctic (and has only one species in the Nearctic). All 190 species in 13 genera are thought to be entomophagous, eating either ants, their regurgitations, or ant-associated hemipterans and their secretions (Fig. 15, video at https://www.youtube.com/watch?v=ZmCz2UxKaHA) (Cottrell 1984; Eliot 1986; Kaliszewska et al. 2015; Pierce 1995). Many adult Miletinae have an especially long, sclerotized abdomen and legs, possibly to protect against the occasional ant bite while alighting and/or ovipositing among hemipteran prey that are being tended by ants (Cottrell 1984; Pierce 1995).
Ants intensively palpate and display interest in the larvae of many Miletinae, but larvae lack dorsal nectary organs, and only species in the Afrotropical genus Aslauga possess tentacle organs (Bascombe et al. 1999; Claassens and Heath 1997; Cottrell 1984; Dejean et al. 2017; Lohman and Samarita 2009; Pierce et al. 2002). Most appear obligately ant-associated (Table 2). Larvae of Oriental and Palearctic Spalgis and Taraka spp. and Nearctic Feniseca tarquinius appear facultatively ant-associated and are protected by silk shelters or cuticular hydrocarbons of ant-attended prey (photos at iNaturalist #57006925 and iNaturalist #14834663) (Cottrell 1984; Lohman et al. 2006; Youngsteadt and Devries 2005). Larvae of F. tarquinius produce vibratory signals that may be ant-related (Mathew et al. 2008).
Kaliszewska et al. (Kaliszewska et al. 2015) found that the subfamily of hemipteran-attending ant is strongly conserved phylogenetically within Miletinae, whereas hemipteran host preference can be quite broad (Fiedler and Maschwitz 1989b; Lohman and Samarita 2009). For example, lycaenids in the genus Miletus appear to associate only with species of ants in the genus Dolichoderus , which adults use to find their hemipteran prey. All 27 species in the southern African genus Thestor are thought to parasitize ants in the genus Anoplolepis (Formicinae), particularly A. custodiens (Claassens and Dickson 1980; Clark and Dickson 1971; Pringle et al. 1994). While the larvae of the majority of Miletinae feed on Hemiptera, later instars may occasionally be carried into the ant nest, where they feed on ant regurgitations and sometimes also ant eggs and detritus (Clark and Dickson 1960; Clark and Dickson 1971; Heath and Claassens 2000; Heath and Claassens 2003; Heath and Pringle 2004; Williams and Joannou 1996).
Caterpillars of the sister genera Liphyra and Euliphyra inhabit the nests of weaver ants in the genus Oecophylla (Fig. 16). Oriental Liphyra brassolis and Liphyra grandis feed voraciously on ant brood and are protected from occasional attack by a thick, bulky “chain link” integument derived from modified setae (Braby 2000; Dupont et al. 2016; Pierce 1995). African species of Euliphyra also coax trophallaxis, intercept trophallaxis between workers, and steal brood within Oecophylla nests (Dejean et al. 2017; Fiedler 2012). Liphyra pupae are entirely enclosed within the hardened exuviae of the last larva instar, or puparium, and Euliphyra pupae only partially emerge during larval-pupal ecdysis (Eltringham 1913). Euliphyra larvae have been shown experimentally to find Oecophylla nests by following ant trail pheromones (Dejean and Beugnon 1996), and Liphyra probably do this as well (Common and Waterhouse 1981; Pierce et al. 2002). Larvae of Liphyra, along with those of Thestor, have short, stubby antennae used to seek and manipulate prey (Dupont et al. 2016). Adult Liphyra are protected upon eclosion by a thick vestiture of greasy, loose scales that slip off in the mandibles of vicious attacking ants, similar to several other species that pupate in ant nests (Atsatt 1981a; Cottrell 1984; Dodd 1902; Hinton 1951; Pierce 1995).
The Miletinae likely constitute the largest radiation of entomophagous lepidopterans (Cottrell 1984; Pierce 1995), and ant association may be linked to the success of this dramatic dietary shift. Body plan constraints may limit the success of predatory caterpillars, except around concentrated food resources or in situations where there are few competing predators (Pierce 1995)—ant brood and ant-attended hemipterans meet both of these conditions. The reverse dietary shift in spiders follows the same principle: the only two spider species with known specializations for plant-feeding are found on well-defended ant-plants with few other herbivores (Meehan et al. 2009; Nyffeler et al. 2016; Painting et al. 2017).
Aphnaeinae
The Aphnaeinae, which along with the Poritiinae are sister to the Miletinae (Espeland et al. 2018), are a largely African subfamily that seems to have been ancestrally associated with Crematogaster ants and legume feeding (Boyle et al. 2015). All species whose life histories are known appear obligately ant-associated (Fig. 17, Table 2). Moreover, at least one species in each of nine genera is aphytophagous, feeding on hemipterans or the eggs, brood, or regurgitations of ants (Boyle et al. 2015; Pierce 1995; Sanetra and Fiedler 1996). One species, Aleiodes pallida , is known to feed in early instars on species of Aspalathus (Fabaceae), but Heath and Claassens (Heath and Claassens 2000) were able to rear final-instar caterpillars of this species in observation nests of the formicine ant, Lepisiota capensis , where caterpillars selectively ate only the ant eggs and not the brood. Additional evidence suggests that several other species of Aloiedes may share this ability to shift from eating plants to eating ant eggs in the final instar. Other species, with exclusively parasitic larval habits, appear in several otherwise phytophagous genera (Basu and Kunte 2020; Fiedler 2012; Heath and Claassens 2003; Pierce 1995). Many Aphnaeinae depend on the presence of a specific species of ant to oviposit (Heath 1997). Dish organs or dew patches are dish-like depressions found on the anterior abdomen in several ant-attended genera of Aphnaeinae that appear to produce reward secretions (Basu and Kunte 2020; Clark and Dickson 1971; Cottrell 1984; Vegliante and Hasenfuss 2012). Several authors note that caterpillars of different species of Aphnaeinae will die if ants are not present to remove secretions from the dew patches and the DNO to prevent them from growing moldy (e.g., Heath 1997; Williams 1995-2020). Tentacle organs of aphnaeine larvae are often housed in protruding cylindrical bases and can be deployed almost like a cat-o’-nine-tails to shoo away overly persistent ants from the DNO (video at https://www.youtube.com/watch?v=Qkd23Pmucmk) (Fiedler 1991).
Poritiinae
The Poritiinae are a subfamily of lycaenid butterflies with non-trophobiotic caterpillars. The approximately 729 species are divided into two clades: the small Asian tribe Poritiini and the large African tribe Liptenini (sometimes split further). Among these, the Liptenini are notable for their lichenivorous diet, although larvae of Deloneura have also been recorded feeding on honeydew near ants (Heath and Claassens 2003; Williams 2006). Larvae of some species feed on lichens growing on bark, rocks, or sticks along the ground and may not be found around ants (Larsen 2005; Williams 2006). While poritiine larvae in the generally open habitats of southern Africa are generally facultatively ant-associated (with the exception of Deloneura ), most species in the wetter forests of West Africa seem to be obligately ant-associated (Bampton 1995). Adults of many species are only found around individual colonies of arboreal Crematogaster ants (Larsen 2005; Sáfián 2015b), and caterpillars of several genera have been reared from ant-infested trees (Callaghan 1992a; Dejean et al. 2017; Jackson 1937; Sáfián 2015a; Sáfián and Collins 2014; Sáfián and Larsen 2009). Over 50% of Poritiinae belong to genera that appear to contain obligate ant associates (Table 2). Obligately ant-associated poritiines tend to be rare, and some species have only ever been found in association with a single arboreal ant colony, raising considerable conservation concern (Larsen 2005; Williams 1995-2020). The lack of obligate ant association in some genera of Poritiinae is perhaps a secondary loss—except for the Poritiini that remain understudied, all major lineages of Poritiinae include species apparently only found on trees along with their associated ant species. Together, the subfamilies Miletinae, Aphnaeinae, and Poritiinae probably constitute the largest single radiation of obligately ant-associated Lepidoptera.
All caterpillars of Poritiinae are covered in long bristles that appear to repel ants (Callaghan 1992b; Dejean et al. 2017). They are probably also chemically defended, as larvae of many species interact with ants with no sign of overt conflict (Farquharson et al. 1922; Sáfián and Collins 2014; Sáfián and Larsen 2009). Ants are repelled from many liptenine caterpillars, perhaps because they secrete toxic chemicals. Some species form large larval aggregations, and others appear to be aposematic (Sáfián 2015a; Sáfián and Larsen 2009). Tussock moth caterpillars (Erebidae: Lymantriinae) protected by defensive glands are sometimes found near poritiine caterpillars in Africa and may similarly be associated with arboreal ant colonies (Farquharson et al. 1922; Hinton 1951). These lymantriine and poritiine caterpillars are visually similar and possibly form a Müllerian mimicry complex (Farquharson et al. 1922).
Riodinidae
Riodinidae are sister to Lycaenidae, and while the 153 genera of Riodinidae are distributed worldwide, more than 1400 species are found in Central and South America. The ca. 120 Old World species are concentrated in Southeast Asia (Espeland et al. 2015; Seraphim et al. 2018). Most Riodinidae are not known to be ant-associated and possess long setae and chemical defenses that prevent ants from getting too close (e.g., Ballmer and Pratt 1988; DeVries 1988a; Fiedler 1991; Kaminski 2008a; Mota et al. 2014; Nishida 2010; Vélez-Arango et al. 2010). Larval aggregation and aposematism are also widespread among riodinids (Fig. 18) (Allen 2010; Callaghan 1986; Janzen and Hallwachs 2021; Nishida 2010). Recorded ant associations are limited to the tribe Nymphidiini and subtribe Eurybiina of the tribe Eurybiini, both of which are in the strictly Neotropical subfamily Riodininae (Espeland et al. 2015). Almost a thousand riodinid species belong to genera that are non-trophobiotic and generally not known to be ant-associated (Table 2).
Eurybiini
In ant-attended larvae of the subtribe Eurybiina of the riodinid tribe Eurybiini, modified TOs, called tentacle nectary organs (TNOs), evert to release a drop of fluid that ants eagerly drink (Horvitz et al. 1987). However, larvae of the subtribe Mesosemiina, sister to the subtribe Eurybiina (Espeland et al. 2018; Seraphim et al. 2018), have never been found with ants, and their TOs are protected by defensive bristles (Nielsen and Kaminski 2018; Vélez-Arango et al. 2010). Nielsen and Kaminski (2018) found that TOs of these larvae evert and extrude a droplet of liquid when attacked by various predators including wasps, biting midges, and lacewing larvae. Ants that came into contact with this liquid cleaned themselves and shunned the larva (Nielsen and Kaminski 2018). Larvae of Symmachiini (Riodininae), which are not ant-associated, also possess tentacle organ openings that may prove to have a similar function (Seraphim et al. 2018).
Nymphidiini
In the riodinid tribe Nymphidiini, all known larvae are ant-associated and typically secrete liquid droplets from glandular tissue within the tentacle organs for ants to imbibe (Fig. 19) (Callaghan 1986; DeVries 1988b; DeVries 1997; DeVries and Penz 2000; Hall and Harvey 2001; Kaminski 2008b; Kaminski and Carvalho-Filho 2012; Kaminski et al. 2016; Kaminski et al. 2013; Mota et al. 2020; Ross 1964; Torres and Pomerantz 2016). The TNOs may be everted most often when the larva is vulnerable or attending ants have only started to arrive (DeVries 1988b).
In a handful of related Nymphiidini, a pair of metathoracic anterior tentacle organs (ATOs) induce alarm in attending ants, sensitizing them to future threats much like the TOs of Lycaenidae (DeVries 1988b, 1997; Kaminski and Carvalho-Filho 2012; Kaminski et al. 2016; Penz and DeVries 2006). Brush-like setae at the apex of the ATOs likely help disperse volatile chemicals (DeVries 1997; Ross 1964). DeVries (1988b) found that the ATOs are important for these larvae to maintain the attention of attending ants and activate most often when the larva is initiating contact or vulnerable. Some phylogenetically earlier-branching Nymphidiini have thoracic PCO clusters that appear homologous in position to the anterior tentacle organs and similarly excite ants (Kaminski et al. 2013).
Balloon setae, swollen structures on the prothorax, may play a role in myrmecophilous interactions in some Nymphidiini that lack ATOs (Kaminski 2008a; Penz and DeVries 2006). However, balloon setae appear to serve a largely defensive function and are shared by many non-myrmecophilous caterpillars (Fig. 20) (Hall et al. 2004; Kaminski et al. 2013; Mota et al. 2014). While Zabuella paucipuncta (Nymphidiini) lacks ATOs, a unique cervical gland that is exposed when ants antennate the balloon setae causes the ants to react in alarm (DeVries et al. 2004).
Adaptations for ant attendance have largely been lost in the riodinid genus Stalachtis (Nymphidiini), but caterpillars remain facultatively ant-associated, and their cuticle appears attractive to diverse ants, much as in some non-trophobiotic lycaenids (Espeland et al. 2015; Seraphim et al. 2018, https://www.flickr.com/photos/142712970@N03/33322969114, https://www.flickr.com/photos/142712970@N03/40459961724, https://www.flickr.com/photos/142712970@N03/38713147222/, https://www.flickr.com/photos/142712970@N03/27298752638/, https://www.flickr.com/photos/142712970@N03/34660951953/, https://www.flickr.com/photos/142712970@N03/48374845847/).
Vibratory signals in ant-attended riodinid larvae are produced through several different mechanisms. Larvae of ant-attended Eurybiini produce sound by rubbing small teeth on the cervical membrane against granulations on the head (DeVries and Penz 2000; Travassos et al. 2008). Larvae of ant-attended Nymphidiini produce sounds using vibratory papillae, small rodlike structures on the prothorax that rub against granulations on the head. Larvae can adjust the beat frequency of the vibratory papillae, with higher rates attracting more ants. Vibratory papillae of Thisbe irenea beat fastest when the larva is stressed, traveling, or during initial contact with ants (DeVries 1988b).
Riodinidae in several genera have independently evolved hemipteran diets (DeVries 1997; Mota et al. 2020). Many species in both Eurybiini and Nymphidiini cohabit with ants, including a single species, Aricoris arenarum , in which the first two instars steal honeydew from ant-attended hemipterans and solicit trophallaxis, and later instars feed by trophallaxis within Camponotus nests (DeVries 1997; Kaminski et al. 2020b; Robbins et al. 1996). Another riodinid caterpillar was recently found preying on ant brood in arboreal nests of Neoponera villosa (Rocha et al. 2020). As in the Lycaenidae, adults of aphytophagous Riodinidae frequently have greasy wings that may help them to escape ants (DeVries 1997; Espeland et al. 2015; Hall and Harvey 2002), and the greasiness of wings has been used to successfully predict larval diet in at least one instance (Hall 2007; Mota et al. 2020). The TNOs no longer secrete rewards in ant-associated hemipterophagous riodinids, but still signal to ants (Kaminski et al. 2020b; Mota et al. 2020), much as nectary organs have been lost in the predatory lycaenid subfamily Miletinae. The predatory larvae of Neotropical Pachythone spp. (Mota et al. 2020) are also remarkably convergent in appearance and adaptive morphology to the ecologically similar larvae of Afrotropical Aslauga spp. (Lycaenidae: Miletinae) (Dejean et al. 2017).
Mutualism and Manipulation: Caterpillar-Ant Trophobiosis in Lycaenidae and Riodinidae
All ant species known to tend trophobiotic caterpillars are agricultural in the sense that they also harvest plant extrafloral nectar and the honeydew produced by Hemiptera (DeVries 1991b; Eastwood and Fraser 1999; Fiedler 2001, 2006; Pierce and Elgar 1985). They include genera such as Iridomyrmex , Oecophylla , Camponotus , and Crematogaster that are among the most dominant ants in the regions where they occur, with wide distributions and large colony sizes that are often polydomous in structure. Caterpillars may take advantage of ant preadaptations to harvest carbohydrate rewards, which are essential resources for ants in many environments (Blüthgen and Fiedler 2002, 2004; Blüthgen et al. 2003; Davidson et al. 2003; Dejean et al. 2007; Grover et al. 2007; Kaspari et al. 2020; Kaspari et al. 2012; Pohl et al. 2016; Ribas and Schoereder 2004). Although biochemically modified to attract ants in many species, hemipteran honeydew is an excrement, produced whether attendant ants are present to collect it or not (Stadler and Dixon 2005). Semutophila saccharopa (Tortricidae) are the only caterpillars that produce sugar-rich excrement for ants in a manner similar to aphids. In contrast, lycaenid and riodinid larvae produce secretions tailored specifically for their ant associates and released from specialized exocrine glands. This distinction is an important one because exocrine glands provide opportunities for lycaenids and riodinids to fine-tune their secretions to manipulate ant behavior, without necessarily providing nutritious rewards.
Ants, although they confer substantial overall benefit to aphid populations, sometimes consume honeydew-producing hemipteran mutualists, particularly when alternative carbohydrate sources are available (Offenberg 2001; Shibao et al. 2009; Silveira et al. 2010; Stadler and Dixon 2005). In contrast, ants have been rarely reported to attack lycaenid larvae except under unnatural circumstances in captivity. Ants suffer a serious opportunity cost when they invest in protecting caterpillars rather than preying on them, especially facultatively ant-attended lycaenids whose secretions may provide poor-quality rewards. The striking absence of overt ant predation also suggests that lycaenid caterpillars must be able to manipulate ants, at least sufficiently to avoid aggression (Fiedler 1998a; Fiedler et al. 1996).
A number of lycaenid larvae mimic the cuticular hydrocarbon (CHC) profiles of host plants or ants or conceal themselves entirely by lacking recognizable molecules (Barbero 2016; Inui et al. 2015; Lima et al. 2020; Lohman 2004; Morozumi et al. 2019), much as reported for different honeydew-producing hemipterans (Endo and Itino 2013; Silveira et al. 2010). This “cloak of invisibility” can ensure that a caterpillar is not attacked by the ants, even if it is not actively tended. CHC mimicry of ants plays an intimate role in the adoption of parasitic species like Phengaris by host ants, as reviewed by Barbero (2016) and Casacci et al. (2019b). Chemical disguise may be observed in other groups when more lycaenid species are studied, but it is clearly not universal (Hojo et al. 2014a; Omura et al. 2009). Other mechanisms might also exist to avoid ant predation. Pupae of facultatively ant-attended Lycaeides argyrognomon , which sometimes cohabit with Camponotus or Formica host ants, have been found to subdue ant aggression through the presence in their cuticle of several long-chained aldehydes not seen in larvae (Mizuno et al. 2018).
DNO and TNO secretions contain amino acids and carbohydrates and have been studied in about ten species (Cushman et al. 1994; Daniels et al. 2005; DeVries 1988b; Pierce and Nash 1999; Pierce et al. 2002; Wada et al. 2001). Secretions of obligate or steadily ant-attended larvae have a higher nutritive content than those of less myrmecophilous species (Daniels et al. 2005).
Aphid mimicry may be one way that facultatively attended larvae attract ants. Melezitose is an aphid gut compound that serves as an attractant for aphid-attending ant workers and is a major component of the nectary secretions in Polyommatus icarus and Zizeeria knysna [Polyommatini], one of the few well-studied species whose caterpillars are weakly attended (Daniels et al. 2005; Depa et al. 2020; Detrain et al. 2010; Vantaux et al. 2011).
Hojo et al. (2015) determined that the facultatively ant-attended Japanese lycaenid Arhopala (=Narathura) japonica produces DNO secretions that manipulate the dopaminergic pathway in the brains of their attendant ants, workers of Pristomyrmex punctatus . Reduced levels of dopamine are correlated with a reduction in worker activity levels (thereby increasing their fidelity to the caterpillar) and heightening aggression toward intruders. Specialized cuticular hydrocarbons (CHCs) of A. japonica act as a signal to host ants that they learn to associate with reward after attending larvae (Hojo et al. 2014a).
The parasitic species Niphanda fusca has larvae that secrete primarily only trehalose and glycine for host Camponotus japonicus ants (Hojo et al. 2008; Wada et al. 2001). Glycine alone is ignored by ants at low concentrations but acts as a manipulative “umami” taste enhancer substance when added to trehalose, increasing host ant interest in this specific sugar (Hojo et al. 2008; Wada et al. 2001). The relative simplicity of this “umami” mechanism for taste enhancement (i.e., the coupling of an amino acid or small peptide with a sugar reward) makes it an attractive candidate for further research into how and why lycaenid caterpillars can be so extremely attractive to their associated ants. However, these same taste preferences are not shared by the closely related Camponotus obscuripes , indicating that lycaenid secretion components may be specialized to individual ant species (Hojo et al. 2008).
By comparing the foraging behavior of colonies of the attendant ant species, Iridomyrmex mayri , fed on high-protein, high-carbohydrate, or mixed diets, Pohl et al. (2016) showed experimentally that the nutritional state of the attendant ant colony influenced the number of attendant workers foraging on larval secretions from the Australian lycaenid, Jalmenus evagoras (Fig. 21). Workers from colonies fed on either carbohydrate- or protein-restricted diets were inconsistent in their compensatory behavior. Those on low-carbohydrate diets compensated by foraging more on sugars, but those on low-protein diets did not show compensatory behavior by foraging more on amino acids. However, workers from colonies that were diet restricted were significantly more interested in foraging on secretions from the larvae than those from well-fed colonies. Workers were not strongly attracted to the amino acid serine, which had been thought to be the primary amino acid in Jalmenus evagoras larval secretions (Pierce 1984) nor did they show an “umami” response when serine was coupled with sugar. More recent analysis suggests that glutamine rather than serine is the primary amino acid in J. evagoras larval secretions (Zemeitat 2017), and further work will be necessary to explore the relationship between larval secretions and ant attendance. The chemical composition of liquid secretions produced by different species of ant-attended larvae varies depending on the species and seems likely to be shaped at least in part by the feeding preferences of the ants that attend each caterpillar species (Daniels et al. 2005; Pierce 1984).
Certain obligate lycaenid-ant associations may provide sufficient fitness benefits to both partners under some conditions to warrant being classified as mutualists (Cushman et al. 1994; Fiedler and Maschwitz 1988; Fiedler et al. 1996). For example, caterpillar secretions from the Australian Jalmenus evagoras confer a net benefit in terms of positive growth rates (potentially resulting in a greater production of alates) for colonies of its most common ant associate, Iridomyrmex mayri (Pierce et al. 1987).
However, even associations with larvae of Jalmenus evagoras may sometimes be detrimental to ants. Under experimental conditions, small ant colonies grew faster when allowed to collect secretions from Jalmenus evagoras larvae, but colonies provided with only one larva grew significantly faster than colonies given access to five larvae, although this may have been because larvae were allowed to aggregate on the host plants (Nash 1990; Pierce and Nash 1999). Subsequent experiments showed that once small groups of workers and brood passed below a minimum ratio of workers to brood, workers would consistently chose to tend J. evagoras larvae and neglect their own brood, allowing them to perish (Merrill 1997; Pierce and Nash 1999). As has been demonstrated for symbioses more generally, whether the relationship is mutualistic or parasitic varies spatially and temporally and is influenced by a number of factors (Madeiros et al. 2018; Thompson 2005; Warren et al. 2019). In the case of caterpillar-ant interactions, this context dependency can include the size of the ant colony, the availability of alternative resources, the number of caterpillars, and the relative cost of their defense.
In certain contexts, manipulation can stabilize mutualisms (Heil et al. 2014; Sachs 2006). However, the manipulation and asymmetry seen in typical lycaenid-ant associations suggest that despite their superficial similarity to honeydew-secreting insects such as aphids, the majority of phytophagous lycaenids might best be viewed as only rarely mutualistically associated with ants and more often mildly parasitic upon them. This association could also help to explain why lycaenids are unusually prone to shifts to overt parasitism and obligate aphytophagy (Sachs and Simms 2006). A number of different ant species are obligately dependent upon associated plants, fungi, and even hemipterans, but it is perhaps significant that none are known to be obligately associated with caterpillars (Chomicki and Renner 2015; Eastwood and Fraser 1999; Ivens 2015).
Because many non-trophobiotic and trophobiotic caterpillars associate with the same species of ants, the primary advantage for trophobiotic caterpillars seems to be that they can attract ants with additional food rewards, not simply that they are able to appease them. There are many reasons why myrmecophilous lycaenids may utilize ants for defense rather than relying on toxic secondary compounds or other means of caterpillar protection. Different circumstances in combination with factors such as larval size or feeding activity can influence predation risk for non-myrmecophilous caterpillars (Berger et al. 2006; Bernays 1997; Dmitriew 2011; Gotthard 2000; Mänd et al. 2007). Selection will favor conditional ant association for protection if maintaining an ant guard is both possible and metabolically less expensive than other means of defense (see discussion below) (Mizuno et al. 2019; Wagner 1993). Different attending ant species can confer significantly different levels of protection (Fraser et al. 2001), but predation or parasitism rates are typically many-fold higher in the absence of ants, both for facultative and obligate ant associates (Atsatt 1981a; Forister et al. 2017; Kaminski et al. 2010a; Rodrigues et al. 2010; Peterson 1993; Pierce and Easteal 1986; Pierce and Mead 1981; Thomas et al. 2020; Weeks 2003). For example, the obligately ant-associated juveniles of the Australian lycaenid, Jalmenus evagoras , suffered nearly 100% mortality from parasites and predators when ants were experimentally removed (Pierce et al. 1987), and even the facultatively attended larvae of the North American lycaenid, Glaucopsyche lygdamus, were shown experimentally in one field season to suffer up to a 12-fold difference in mortality without ants (Pierce and Easteal 1986).
Highly specialized parasitoids and predators may seek out myrmecophilous caterpillars by using chemical or vibrational cues from their associated ants to locate their prey (e.g., Dejean et al. 2016; DeVries 1991b; Elgar et al. 2016; Fiedler et al. 1992; Pierce and Nash 1999; Thomas and Elmes 1993; Thomas et al. 2002). In situations like this where specialized enemies use attendant ants to find their prey, selection might favor the loss of ant association. Ant associations in plants and insects are prone to frequent loss or modification, and Lepidoptera are no exception (Chomicki and Renner 2015; Sachs and Simms 2006; Stadler and Dixon 2005; Weber and Keeler 2013; Yao 2014). For example, several Australian species in the obligately ant-associated lycaenid genus Hypochrysops have likely either lost ant association (Hypochrysops byzos , H. pythias ) or become facultatively associated with them (H. polycletus ) (Braby 2000). In another Australian genus, Ogyris , while most species have caterpillars that are never found without ants, often with only a single genus or species, the clade containing Ogyris amaryllis , O. oroetes , O. olane , and O. barnardi has lost or greatly reduced facultative ant associations (Eastwood and Fraser 1999; Schmidt and Rice 2002). The Taraka-Spalgis-Feniseca clade (Lycaenidae: Miletinae) and several genus-groups of Poritiinae represent other cases where phylogenetic studies will probably reveal extensive secondary loss of obligate ant associations. Ant associations appear lost most frequently in facultatively ant-associated groups of the Theclinae-Polyommatinae assemblage, a pattern also observed in facultative associations more generally (Chomicki et al. 2020).
Like many classic symbioses, ant-caterpillar associations typically have additional hidden partners. Hemipteran-ant mutualisms often benefit host plants (Campbell et al. 2013; Pringle et al. 2011; Styrsky and Eubanks 2007), and host plants may similarly benefit when lycaenid and riodinid caterpillars attract ants that drive away other herbivores and deposit nutrients, as recently documented for Euchrysops cnejus caterpillars attended by Camponotus ants on Vigna plants in India (Ekka et al. 2020).
Abiotic Effects, Obligate Associations, and Biogeography
One of the more significant insights gained in recent years from a worldwide consideration of the drivers of interspecies symbiosis (e.g., Kaspari 2020; Steidinger et al. 2019) is the importance of abiotic factors in determining the distribution of species interactions such as those seen between caterpillars and ants. Pierce (1987) pointed out a striking pattern in the biogeographic distribution of lycaenid-ant interactions: obligate interactions are considerably more common in the Southern Hemisphere, particularly Australia and Southern Africa, compared to those in the Northern Hemisphere, including the Nearctic and Palearctic. These patterns appear to extend into wet tropical Africa and Southeast Asia, where life histories of Lycaenidae are comparatively less well-documented. All but one tribe of lycaends has representatives in both hemispheres, and thus this pattern cannot be explained by a single vicariance event involving ant-associated and non-associated lineages. Rather, it is due to a heterogenous distribution of tribes with different levels of ant association, with Poritiinae, Aphnaeinae, Miletinae, and strongly ant-associated genera of Theclinae-Polyommatinae generally limited to the Afrotropical, Oriental, and Australasian regions. In the same way, ant-associated Riodinidae and other Lepidoptera are almost entirely limited to the Neotropical, Afrotropical, Oriental, and Australasian regions (Table 1).
Two nonexclusive explanations for this pattern include (1) climate differences and (2) bottom-up effects of soil micronutrients and precipitation that affect plants, microbes, and species that interact with them (e.g., Steidinger et al. 2019). For example, the phosphorus-poor soils of southern Africa and Australia have been evoked as potentially playing a role in the high percentage of ant-dispersed myrmecochorous plants in these areas (Westoby et al. 1982). Research has accumulated over the past 25 years in what Kaspari has called “ionic ecology” (Kaspari 2020), demonstrating the importance of the stoichiometry of essential elements like Na, P, Cl, K, Mg, and Ca that flux across membranes of organisms at different trophic levels.
Functional mechanisms involving different species are complex and undoubtedly vary depending upon the circumstances, but numerous experiments at a variety of spatial scales focusing on invertebrates ranging from termites (with a rich gut microbiome) to caterpillars (largely devoid of a gut microbiome) have shown that levels of accessible environmental sodium either from direct access through soil or “mud puddles” or indirectly through plant tissues consumed by herbivores can have an enormous effect not only on the abundance and distribution of invertebrates but also on the entire network of parasites and predators interacting with them (Baker et al. 2020; Kaspari 2020). The significance of these results in considering caterpillar-ant interactions seems especially clear given the trophobiotic nature of most of their associations. Coping mechanisms are required in habitats with soils that are poor in phosphorus: an essential, rare, and limited nutrient needed for ribosomes, ATP, and nucleic acids. For insects with gut microbiota, movement into the cell can be facilitated by bacteria with surface proteins that can cotransport Na-P across membranes (Werner and Kinne 2001). This means that sodium can be at a premium for organisms with microbial associations like ants because of its role in facilitating cotransport of phosphates into cells of their symbionts (Kaspari 2020). A growing body of research has shown that the availability of sodium and phosphorus can place constraints on ant growth (Bujan et al. 2016; Goitía and Jaffé 2009; Kaspari et al. 2008, 2009, 2020). Any mechanism that could enhance sodium acquisition and/or facilitate sodium ion transport might be especially favored in regions where soils have low phosphorus or sodium.
For plants, this could be achieved through extrafloral or floral nectars or through seeds with attractive eliasomes. For caterpillars, this could perhaps be achieved with secretions and could explain the appearance of obligate, intense ant associations in arid habitats with low phosphorus soils such as those found in central Australia and Southern Africa. The same ability to attract and manipulate ant partners would not exist in habitats with well-fertilized soils because ants might not be limited by essential micronutrients in the same way. This difference in soil fertility could also help to explain in part why ant plants are restricted to the tropics. For example, although the genus Macaranga is widely distributed in the Old World tropics, the clade containing ant plants occurs in West Melanesian rainforest, a region also characterized by phosphorus-poor soils (Davies et al. 2001). Similarly, natural variation as well as experimental manipulations in nutrient exposure of obligately associated ant plants ranging from Cordia and Cecropia in the Neotropics to Macaranga and Vachellia in the Old World tropics have resulted in differences in plant growth rates and turnover of ant inhabitants (Folgarait and Davidson 1995; Heil et al. 2001; Pringle et al. 2013).
Aridification has played an additional role in affecting ant associations, both by driving caterpillars to seek shelter and possibly food in ant nests (Espeland et al. in review) and by generating extremes in the distribution of soil micronutrients over a large spatial scale (Bui et al. 2014). It seems no coincidence that relatively high levels of ant association are observed in caterpillar-ant interactions in Australia, Southern Africa, and the Cerrado of Brazil.
Finally, differences in soil composition across large spatial scales may have also played a more important role in shaping ant association across landscapes of the Southern Hemisphere including Australia and Southern Africa because weathering and erosion processes have taken place in the absence of the kind of severe, cyclical history of glaciation observed in the Northern Hemisphere (Hopper 2009). This explanation has the advantage of accounting for different levels of obligate association in similar temperate climates of the Western Palearctic (Fiedler 1998b) versus in Australia (Pierce 1987).
Experimental evidence suggests that seasonal temperature fluctuations can break up established partnerships by disrupting ant ecological partitioning. Ant territorialism on caterpillar host plants seems to facilitate obligate ant-caterpillar association, as exemplified by species of aggressive, tropical, arboreal Oecophylla and Crematogaster found with diverse specialized Lepidoptera. Ecologically dominant ant species typically have the most abundant and aggressive workers, and their large colonies create stable, high-quality habitats for myrmecophiles (Eastwood and Fraser 1999; Fiedler 1991; Fiedler 2001; Fiedler 2006; Hölldobler and Wilson 1990). The combined suitability of a host plant to feed on and enemy free space afforded by the ability to appease otherwise threatening ants can create “ecological islands” of opportunity for obligately specialized myrmecophilous Lepidoptera, and this can have strong effects on their subsequent diversity through restriction of population size and/or structuring of populations (Eastwood et al. 2006; Pellissier et al. 2012; Pierce et al. 2002; Schär et al. 2018). This helps explain why New World Eciton and Old World Dorylus army ant species, although the former have the most diverse myrmecophile communities known, do not have caterpillar associates—their lepidopteran interactions are restricted to a few species of Papilionidae, Hesperiidae, and Nymphalidae whose adults use ant columns to find nitrogen-rich bird droppings (Ivens et al. 2016; Kistner 1982; Rettenmeyer et al. 2011). The foraging strategies of army ants afford little opportunity for caterpillars, which are relatively sedentary and typically herbivores, to form stable associations (Pierce 1995; Powell et al. 1998). Ant partners in temperate regions might also be less desirable due to the various documented effects of climate on colony traits and community structure (Dunn et al. 2010; Dunn et al. 2009; Kaspari and Vargo 1995; Kaspari et al. 2000).
Besides making suitable ant partners difficult to find, climate fluctuations in temperate areas might increase caterpillar developmental times and reduce access to nutritious host plants (Fiedler 2006; Pellissier et al. 2012a). These hypotheses are supported by the observation that obligate ant associates in temperate areas generally spend most of their life cycle within ant nests, where nutritious food sources, favorable microclimates, and attendant ants are always available (Fiedler 2006). Caterpillar life histories are generally more specialized and diverse in the tropics (Dyer et al. 2007; Forister et al. 2015). Further studies are needed to elucidate the underlying ecological causes.
Whether based on climate, soils, the availability of required ants, or other factors, only non-myrmecophilous or flexible, facultative ant associates in the families Lycaenidae and Riodinidae are found extensively beyond the Old World and New World southern zoogeographic regions (Fig. 22). The Lycaenidae are thought to have originated in the Old World tropics, where extant phylogenetic diversity remains heavily concentrated (Espeland et al. 2018). Only five lycaenid groups occur substantially beyond the Old World tropics and subtropics into the Palearctic, and all five have also entered the New World through Beringia or across the Atlantic (Fric et al. 2019; Gompert et al. 2008; Vila et al. 2011): the Spalgis -Taraka -Feniseca clade [Miletinae], the subfamily Lycaeninae, and the tribes Theclini, Eumaeini, and Polyommatini of the Theclinae-Polyommatinae assemblage. Except for a handful of obligately associated species of Polyommatini found within otherwise facultatively associated genus groups, species in these groups are all largely facultatively ant-associated or not ant-associated (Table 2, Fig. 23). The Riodinidae have diversified primarily in the Neotropics. A single lineage within the subfamily Nemeobiinae has colonized the Old World, most likely via Beringia, with a secondary return to the Neotropics of the genera Styx and Corrachia (Espeland et al. 2015). None of the over 300 species in this subfamily are known to be ant-associated (Table 2). It is tempting to conclude that strongly ant-associated butterflies have been unable to disperse between the Old World and the New World due to the challenge of finding suitable ant partners, especially when confronted with climatic conditions found in temperate regions.
Geographical distribution of ant associations in Lycaenidae. Known and inferred lycaenid life histories reported in Table 2, or the lack thereof, were cross-referenced with regional catalogs in (Braby 2000; Ek-Amnuay 2012; van Gasse 2013; Hardy and Lawrence 2017; Lamas et al. 2004; Opler 1992, 1999; Parsons 1999; Seki et al. 1991; Tshikolovets 2011; Williams 1995-2020). Facultative associates are often more widely distributed than obligate associates, and they may comprise an increasingly large fraction of total species when looking at small, disturbed, or isolated regions
Phylogenetic tree of extant Lycaenidae and Riodinidae based on data from Espeland et al. (Espeland et al. 2018), showing (a) distribution of species with larval ant association overall, (b) distribution of facultative larval ant associations, and (c) distribution of obligate larval ant associations (from data summarized in Table 2). The size of each circle is proportional to the number of species represented. Percentages correspond to the number of records known or inferred from congeners divided by the total number of species, with groups where life history information such as degree of obligacy is unavailable scored as 0%. Tribes that were rendered non-monophyletic appear multiple times in the tree (e.g., the Polyommatini). We have illustrated the same species counts and ant association proportions next to each appearance as current phylogenies do not allow us to break down these data further. (Figure prepared by João Tonini)
Obligate myrmecophiles’ double reliance on associated ants and food sources may make them especially sensitive to disturbance and vulnerable to extinction (Chomicki et al. 2020; Geyle et al. 2021; Koh et al. 2004; Pierce 1995; Pierce et al. 2002). Invasive ant species, habitat disturbance and destruction, and climate change are particular concerns (e.g., Braby et al. 2021; Geyle et al. 2021). Over 60% of the threatened Lycaenidae and Riodinidae on the IUCN Red List are recorded as obligate ant associates or predicted to be obligate ant associates based on congeners (Table 2) (IUCN 2020). Among the obligately ant-associated species, those that are “aphytophagous,” having at least one life stage obligately dependent on animal rather than plant tissue for nutrition, are particularly vulnerable. Species of Lepidoptera with this rare life history trait comprise only 400 species at most, representing only about 0.25% of the estimated 160,000 species (Pierce 1995). However, aphytophagous species are greatly overrepresented on the IUCN Red List of Threatened Species of butterflies, appearing almost two orders of magnitude more likely to be included in the categories of “Extinct,” “Critically Endangered,” and “Endangered” than are herbivorous species (IUCN 2020). Most obligate ant associations remain poorly studied. Moreover, countless groups of ant-associated caterpillars likely remain undiscovered, as nearly two-thirds of currently known myrmecophile groups are known from only a single species, almost entirely in the tropics (Table 1). Surprising and unique forms of ant association continue to be described regularly (e.g., Agassiz and Kallies 2018; Dejean et al. 2017; Komatsu and Itino 2014; Ramos et al. 2018; Rocha et al. 2020). Future experiments will need to measure the abundance and distribution of myrmecophiles in different regions and habitats to let us estimate their true global diversity, document their often-unbelievable biology, and ensure their future.
Change history
12 July 2022
“The Natural History of Caterpillar-Ant Associations” was previously published non-open access. It has now been changed to open access under a CC BY 4.0 license and the copyright holder updated to ‘The Author(s)’. The book has also been updated with this change.
References
Adams RM, Wells RL, Yanoviak SP, Frost CJ, Fox EG (2020) Interspecific eavesdropping on ant chemical communication. Front Ecol Evol 8:24
Adamski D (2017) A New species of Calosima Dietz, 1910 from Kenya (Lepidoptera: Gelechioidea: Blastobasidae) reared from the Domatia of Vachellia drepanolobium (Fabaceae). Proc Entomol Soc Wash 119(sp1):697–702
Agassiz DJ (2011) The Lepidoptera of acacia domatia in Kenya, with description of two new genera and six new species. J Nat Hist 45(29–30):1867–1893
Agassiz DJ, Bidzilya OV (2016) Gelechiidae (Lepidoptera) bred from acacia in Kenya with description of eight new species. Ann Ditsong Nat Mus Nat Hist 6(07):116–145
Agassiz DJ, Harper DM (2009) The Macrolepidoptera fauna of acacia in the Kenyan Rift Valley (part 1). Trop Lepidop Res 1:4–8
Agassiz DA, Kallies AX (2018) A new genus and species of myrmecophile clearwing moth (Lepidoptera: Sesiidae) from East Africa. Zootaxa 4392(3):588–594
Agrawal AA, Fordyce JA (2000) Induced indirect defence in a lycaenid-ant association: the regulation of a resource in a mutualism. Proc R Soc Lond Ser B Biol Sci 267:1857–1861
Ahn N, Bae Y, Kang E (2014) A first record of Gaphara conspersa (Matsumura, 1931) (Lepidoptera, Tineidae) in Korea. Reg Gen Meet Korean Soc Appl Insects Spring Confer. Accessed at https://www.semanticscholar.org in February 2021
Aibar-Abregú P (2014) Scientific note: hostplant records for the myrmecophilous butterfly Harveyope densemaculata (Hewitson, 1870)(Lepidoptera: Riodinidae). Trop Lepidop Res 24(2):121
Akino T, Knapp JJ, Thomas JA, Elmes GW (1999) Chemical mimicry and host specificity in the butterfly Maculinea rebeli, a social parasite of Myrmica ant colonies. Proc R Soc Lond Ser B Biol Sci 266:1419–1426
Akino T, Nakamura KI, Wakamura S (2004) Diet-induced chemical phytomimesis by twig-like caterpillars of Biston robustum Butler (Lepidoptera: Geometridae). Chemoecology 14(3–4):165–174
Albanese GE, Nelson MW, Vickery PD, Sievert PR (2007) Larval feeding behavior and ant association in frosted elfin, Callophrys irus (Lycaenidae). J Lepidop Soc 61(2):61–66
Allen PE (2010) Group size effects on survivorship and adult development in the gregarious larvae of Euselasia chrysippe (Lepidoptera, Riodinidae). Insect Soc 57(2):199–204
Als TD, Vila R, Kandul NP, Nash DR, Yen SH, Hsu YF, Mignault AA, Boomsma JJ, Pierce NE (2004) The evolution of alternative parasitic life histories in large blue butterflies. Nature 432(7015):386–390
Alves-Silva E, Bächtold A, Del-Claro K (2018) Florivorous myrmecophilous caterpillars exploit an ant–plant mutualism and distract ants from extrafloral nectaries. Austral Ecol 43(6):643–650
Atsatt PR (1981a) Lycaenid butterflies and ants: selection for enemy-free space. Am Nat 118(5):638–654
Atsatt PR (1981b) Ant-dependent food plant selection by the mistletoe butterfly Ogyris amaryllis (Lycaenidae). Oecologia 48:60–63
Austin GT, Boyd BM, Murphy DD (2008) Euphilotes ancilla (Lycaenidae) in the Spring Mountains, Nevada: more than one species. J Lepidop Soc 62(3):148–160
Axén AH (2000) Variation in behavior of lycaenid larvae when attended by different ant species. Evol Ecol 14(7):611–625
Axen AH, Pierce NE (1998) Aggregation as a cost-reducing strategy for lycaenid larvae. Behav Ecol 9(2):109–115
Axén AH, Leimar O, Hoffman V (1996) Signalling in a mutualistic interaction. Anim Behav 52(2):321–333
Ayre GL (1958) Notes on insects found in or near nests of Formica subnitens Creighton (Hymenoptera: Formicidae) in British Columbia. Insect Soc 5(1):1–7
Bächtold A, Alves-Silva E (2013) Behavioral strategy of a lycaenid (Lepidoptera) caterpillar against aggressive ants in a Brazilian savanna. Acta Ethologica 16(2):83–90
Bächtold A, Alves-Silva E, Kaminski LA, Del-Claro K (2014) The role of tending ants in host plant selection and egg parasitism of two facultative myrmecophilous butterflies. Naturwissenschaften 101(11):913–919
Baker CC, Bittleston LS, Sanders JG, Pierce NE (2016) Dissecting host-associated communities with DNA barcodes. Philos Trans R Soc B Biol Sci 371(1702):2015.0328
Baker CC, Castillo Vardaro JA, Doak DF, Pansu J, Puissant J, Pringle RM, Tarnita CE (2020) Spatial patterning of soil microbial communities created by fungus-farming termites. Mol Ecol 29(22):4487–4501
Bálint Z, Benyamini D (2001) Taxonomic notes, faunistics and species descriptions of the austral south American polyommatine lycaenid genus Pseudolucia (Lepidoptera: Lycaenidae): the chilensis and collina species-groups. Annls Hist Nat Mus Nat Hung 93:107–149
Ballmer GR (2008) Life history of Purlisa gigantea (Lepidoptera: Lycaenida, Theclini) in South Thailand. Trop Lepidop Res 1:32–39
Ballmer GR (2015) Pursued by adrenalin. In: Pursuit of dopamine. The lives of lepidopterists. Springer, pp 41–48
Ballmer GR, Pratt GF (1988) A survey of the last instar larvae of the Lycaenidae (Lepidoptera) of California. J Res Lepidop 27(1):1–81
Ballmer GR, Pratt GF (1991) Quantification of ant attendance (myrmecophily) of lycaenid larvae. J Res Lepidop 30(1–2):95–112
Ballmer GR, Wright DM (2008) Life history and larval chaetotaxy of Ahmetia achaja (Lepidoptera, Lycaenidae, Lycaeninae, Theclini, Cheritrina). Zootaxa 1845(1):47–59
Bampton I (1995) A discussion on the larval food of the subfamily Lipteninae (Lepidoptera, Lycaenidae). Metamorphosis 6(4):162–166
Barbero F (2016) Cuticular lipids as a cross-talk among ants, plants and butterflies. Int J Mol Sci 17(12):1966
Barbero F, Bonelli S, Thomas JA, Balletto E, Schönrogge K (2009a) Acoustical mimicry in a predatory social parasite of ants. J Exp Biol 212(24):4084–4090
Barbero F, Thomas JA, Bonelli S, Balletto E, Schönrogge K (2009b) Queen ants make distinctive sounds that are mimicked by a butterfly social parasite. Science 323(5915):782–785
Barbero F, Patricelli DA, Witek MA, Balletto E, Casacci LP, Sala MA, Bonelli S (2012) Myrmica ants and their butterfly parasites with special focus on the acoustic communication. Psyche 2012. https://doi.org/10.1155/2012/725237
Bascombe MJ, Johnston G, Bascombe FS (1999) The butterflies of Hong Kong. Academic, San Diego
Basu DN, Kunte K (2020) Tools of the trade: MicroCT reveals native structure and functional morphology of organs that drive caterpillar–ant interactions. Sci Rep 10(1):1–7
Basu DN, Churi P, Soman A, Sengupta A, Bhakare M, Lokhande S, Bhoite S, Huertas B, Kunte K (2019) The genus Tarucus Moore, [1881] (Lepidoptera: Lycaenidae) in the Indian subcontinent. Trop Lepidop Res 29(2):87–110
Baylis M, Pierce NE (1991) The effect of host-plant quality on the survival of larvae and oviposition by adults of an ant-tended lycaenid butterfly, Jalmenus evagoras. Ecol Entomol 16:1–9
Baylis M, Pierce NE (1992) Lack of compensation by final instar larvae of the myrmecophilous lycaenid butterfly, Jalmenus evagoras, for the loss of nutrients to ants. Physiol Entomol 17:107–114
Benyamini D (1995) Synopsis of biological studies of the Chilean Polyommatini (Lepidoptera, Lycaenidae). Reports of the Museum of Natural History, vol 52. University of Wisconsin, Stevens Point, pp 1–51
Benyamini D (2013) Pseudolucia balinti sp. n. of the plumbea-sibylla species group in central-West Argentina (Lepidoptera: Lycaenidae: Polyommatinae). Fol Entomol Hung 74:157–174
Benyamini D, Bálint Z (1995) Studies of life history and myrmecophily in certain Chilean Pseudolucia Nabokov (Lepidoptera, Lycaenidae). Reports of the Museum of Natural History, vol 51. University of Wisconsin, Stevens Point, pp 1–7
Benyamini D, Aristophanous M, Aristophanous A, John E (2018) The biology of the Cyprus endemic blue Glaucopsyche paphos Chapman, 1920 (Lepidoptera: Lycaenidae, Polyommatinae). Entomol Gaz 69:151–165
Benyamini D, Mega NO, Romanowski HP, Moser A, Vila R, Bálint Z (2019) Distribution, life history and conservation assessment of the critically endangered butterfly Pseudolucia parana (Lepidoptera: Lycaenidae). Fol Entomol Hung 80:303–325
Berger D, Walters R, Gotthard K (2006) What keeps insects small?—size dependent predation on two species of butterfly larvae. Evol Ecol 20:575–589
Bernays EA (1997) Feeding by lepidopteran larvae is dangerous. Ecol Entomol 22:121–123
Bertone MA, Leong M, Bayless KM, Malow TL, Dunn RR, Trautwein MD (2016) Arthropods of the great indoors: characterizing diversity inside urban and suburban homes. PeerJ 4:e1582
Bily S, Fikacek M, Sipek P (2008) First record of myrmecophily in buprestid beetles: immature stages of Habroloma myrmecophila sp. nov.(Coleoptera: Buprestidae) associated with Oecophylla ants (Hymenoptera: Formicidae). Insect Syst Evol 39:1399–1560
Blüthgen N, Fiedler K (2002) Interactions between weaver ants Oecophylla smaragdina, homopterans, trees and lianas in an Australian rain forest canopy. J Anim Ecol 71(5):793–801
Blüthgen N, Fiedler K (2004) Competition for composition: lessons from nectar-feeding ant communities. Ecology 85(6):1479–1485
Blüthgen N, Stork NE (2007) Ant mosaics in a tropical rainforest in Australia and elsewhere: a critical review. Austral Ecol 32(1):93–104
Blüthgen N, Gebauer G, Fiedler K (2003) Disentangling a rainforest food web using stable isotopes: dietary diversity in a species-rich ant community. Oecologia 137(3):426–435
Borges ED, Borges ME, Zarbin PH (2014) Defensive behavior associated with secretions from the prosternal paired glands of the larvae of Heliconius erato phyllis Fabricius (Lepidoptera, Nymphalidae). Rev Bras Entomol 58(2):161–167
Boyle JH, Kaliszewska ZA, Espeland M, Suderman TR, Fleming J, Heath A, Pierce NE (2015) Phylogeny of the Aphnaeinae: myrmecophilous African butterflies with carnivorous and herbivorous life histories. Syst Entomol 40(1):169–182
Braby MF (2000) Butterflies of Australia: their identification, biology and distribution. CSIRO Publishing, Melbourne
Braby MF (2011) New larval food plant associations for some butterflies and diurnal moths (Lepidoptera) from the Northern Territory and eastern Kimberley, Australia. Beagle Rec Mus Art Gall North Territory 27:85–105
Braby MF (2012) New larval food plants and biological notes for some butterflies (Lepidoptera: Papilionoidea) from eastern Australia. Aust Entomol 39(2):65–68
Braby M (2015) New larval food plant associations for some butterflies and diurnal moths (Lepidoptera) from the Northern Territory and Kimberley, Australia. Part II. Rec West Aust Mus 30:73–97. https://doi.org/10.18195/issn.0312-3162.30(2).2015.073-097
Braby MF, Douglas F (2004) The taxonomy, ecology and conservation status of the Golden-rayed blue: a threatened butterfly endemic to western Victoria, Australia. Biol J Linn Soc 81(2):275–299
Braby MF, Williams MR, Douglas F, Beardsell C, Crosby DF (2021) Changes in a peri-urban butterfly assemblage over 80 years near Melbourne, Australia. Aust Entomol 60:27–51
Brandl R, Bagine RN, Kaib M (1996) The distribution of Schedorhinotermes lamanianus (Isoptera: Rhinotermitidae) and its termitophile Paraclystis (Lepidoptera: Tineidae) in Kenya: its importance for understanding east African biogeography. Glob Ecol Biogeogr Lett 5(3):143–148
Bui EN, González-Orozco CE, Miller JT (2014) Acacia, climate, and geochemistry in Australia. Plant Soil 381(1–2):161–175
Bujan J, Wright SJ, Kaspari M (2016) Biogeochemical drivers of Neotropical ant activity and diversity. Ecosphere 7(12):e01597
Bura VL, Fleming AJ, Yack JE (2009) What’s the buzz? Ultrasonic and sonic warning signals in caterpillars of the great peacock moth (Saturnia pyri). Naturwissenchaften 96(6):713–718
Bura VL, Rohwer VG, Martin PR, Yack JE (2011) Whistling in caterpillars (Amorpha juglandis, Bombycoidea): sound-producing mechanism and function. J Exp Biol 214(1):30–37
Burghardt F, Fiedler K (1996) The influence of diet on growth and secretion behaviour of myrmecophilous Polyommatus icarus caterpillars (Lepidoptera: Lycaenidae). Ecol Entomol 21(1):1–8
Bury JA, Savchuk VL (2015) New data on the biology of ten lycaenid butterflies (Lepidoptera: Lycaenidae) of the genera Tomares Rambur, 1840, Pseudophilotes Beuret, 1958, Polyommatus Latreille, 1804, and Plebejus Kluk, 1780 from the Crimea and their attending ants (Hymenoptera: Formicidae). Act Entomol Siles 23:1–6
Callaghan CJ (1985) Notes on the biology of Stalachtis susanna (Lycaenidae: Riodininae) with a discussion of riodinine larval strategies. J Res Lepidop 24(3):258–263
Callaghan CJ (1986) Restinga butterflies: biology of Synargis brennus (Stichel) (Riodinidae). J Lepidop Soc 40(2):93–96
Callaghan CJ (1992a) Notes on the biology of a myrmecophilous African lycaenid, Aphnaeus adamsi Stempffer (Lepidoptera, Lycaenidae). Bull Soc Entomol France 97(4):339–342
Callaghan CJ (1992b) Biology of epiphyll feeding butterflies in a Nigerian cola forest (Lycaenidae: Lipteninae). J Lepidop Soc 46(3):203–214
Callaghan CJ (1997) The biology of Abisara neophron neophron (Hewitson, 1860) from Nepal (Lepidoptera, Riodinidae). Bull Soc Entomol France 2:129–132
Callaghan CJ (2003) The biology of Melanis leucophlegma (Stichel, 1910) (Riodinidae) in western Peru. J Lepidop Soc 57(3):193–196
Callaghan CJ (2008) The biology of Rhamma arria in Colombia (Lepidoptera: Lycaenidae). Trop Lepidop Res 18(1):60–61
Camarota F, Vasconcelos HL, Marquis RJ, Powell S (2020) Revisiting ecological dominance in arboreal ants: how dominant usage of nesting resources shapes community assembly. Oecologia 194(1):151–163
Campbell DL, Pierce NE (2003) Phylogenetic relationships of the Riodinidae: implications for the evolution of ant association. In: Butterflies: ecology and evolution taking flight. University of Chicago Press, Chicago, pp 395–408
Campbell H, Townsend IR, Fellowes MD, Cook JM (2013) Thorn-dwelling ants provide antiherbivore defence for camelthorn trees, Vachellia erioloba, in Namibia. Afr J Ecol 51(4):590–598
Carleial S, Maurel N, van Kleunen M, Stift M (2018) Oviposition by the mountain Alcon blue butterfly increases with host plant flower number and host ant abundance. Bas Appl Ecol 28:87–96
Casacci LP, Schönrogge K, Thomas JA, Balletto E, Bonelli S, Barbero F (2019a) Host specificity pattern and chemical deception in a social parasite of ants. Sci Rep 9(1):1–10
Casacci LP, Bonelli S, Balletto E, Barbero F (2019b) Multimodal signaling in myrmecophilous butterflies. Front Ecol Evol 7:454
Casagrande M, Penz C, DeVries PJ (2009) Description of early stages of Chorinea licursis (Fabricius) (Riodinidae). Trop Lepidop Res 19(2):89–93
Castillo Guevara C, Rico Gray V (2002) Is cycasin in Eumaeus minyas (Lepidoptera: Lycaenidae) a predator deterrent? Interciencia 27(9):465–470
Chialvo CH, Chialvo P, Holland JD, Anderson TJ, Breinholt JW, Kawahara AY, Zhou X, Liu S, Zaspel JM (2018) A phylogenomic analysis of lichen-feeding tiger moths uncovers evolutionary origins of host chemical sequestration. Mol Phylogenet Evol 121:23–34
Chomicki G, Renner SS (2015) Phylogenetics and molecular clocks reveal the repeated evolution of ant-plants after the late Miocene in Africa and the early Miocene in Australasia and the Neotropics. New Phytol 207(2):411–424
Chomicki G, Kiers ET, Renner SS (2020) The evolution of mutualistic dependence. Annu Rev Ecol Evol Syst 51:409–432
Claassens AJ (1996) Notes on the feeding habits and protective measures of the larvae of Durbaniopsis saga van Son (Lepidoptera: Lycaenidae). Metamorphosis 7(3):127–128
Claassens AJM, Dickson CGC (1980) Butterflies of the Table Mountain range. C. Struik, Cape Town
Claassens AJ, Heath A (1997) Notes on the myrmecophilous early stages of two species of Thestor Hübner (Lepidoptera: Lycaenidae) from South Africa. Metamorphosis 8(2):56–60
Clark GC, Dickson CGC (1960) The life- histories of two species of Thestor (Lepidoptera: Lycaenidae). J Entomol Soc S Afr 23:278–283
Clark GC, Dickson CGC (1971) Life histories of the south African lycaenid butterflies. Purnell, Cape Town
Cock MJ (2010) A note on the biology of Pirascca sagaris sagaris (Cramer) (Lepidoptera: Riodinidae) in Trinidad, West Indies. Living World J Trinidad Tobago Field Nat Club 2010:85–86
Collier N (2007) Identifying potential evolutionary relationships within a facultative lycaenid-ant system: ant association, oviposition, and butterfly-ant conflict. Insect Sci 14(5):401–409
Common IFB, Waterhouse DF (1981) Butterflies of Australia, 2nd edn. Angus and Robertson, Sydney
Comstock JA, Dammers CM (1932) Early stages of Melitaea leanira wrightii Edw. and Calephelis nemesis Edw. (Lepidoptera). Bull S Cal Acad Sci 31:9–15
Cottrell CB (1984) Aphytophagy in butterflies: its relationship to myrmecophily. Zool J Linnean Soc 80(1):1–57
Cushing PE (1997) Myrmecomorphy and myrmecophily in spiders: a review. Fl Entomol 80(2):165–193
Cushing PE (2012) Spider-ant associations: an updated review of myrmecomorphy, myrmecophily, and myrmecophagy in spiders. Psyche 2012:151989
Cushman JH, Rashbrook VK, Beattie AJ (1994) Assessing benefits to both participants in a lycaenid-ant association. Ecology 75(4):1031–1041
Czekes Z, Markó B, Nash DR, Ferencz M, Lázár B, Rákosy L (2014) Differences in oviposition strategies between two ecotypes of the endangered myrmecophilous butterfly Maculinea alcon (Lepidoptera: Lycaenidae) under unique syntopic conditions. Insect Conserv Divers 7:122–131
Daniels H, Gottsberger G, Fiedler K (2005) Nutrient composition of larval nectar secretions from three species of myrmecophilous butterflies. J Chem Ecol 31(12):2805–2821
Dantchenko AV (1997) Notes on the biology and distribution of the damone and damocles species-complexes of the subgenus Polyommatus (Agrodiaetus) (Lepidoptera: Lycaenidae). Nachr Entomol Ver Apollo Suppl 16:23–42
Darling CD, Schroeder FC, Meinwald J, Eisner M, Eisner T (2001) Production of a cyanogenic secretion by a thyridid caterpillar (Calindoea trifascialis, Thyrididae, Lepidoptera). Naturwissenschaften 88(7):306–309
Davidson DW, Cook SC, Snelling RR, Chua TH (2003) Explaining the abundance of ants in lowland tropical rainforest canopies. Science 300(5621):969–972
Davies SJ, Lum SKY, Chan R, Wang LK (2001) Evolution of myrmecophytism in West Malesian Macaranga (Euphorbiaceae). Evolution 55:1542–1559
Davis DR, Davis MM (2007) Neotropical Tineidae, V: a new genus and species of Tineidae associated with social hymenoptera and re-examination of two poorly known genera with similar biology (Lepidoptera: Tineidae, Lyonetiidae). Proc Entomol Soc Wash 109(4):741–764
Davis DR, Quintero DA, Cambra RA, Aiello A (2008) Biology of a new Panamanian bagworm moth (Lepidoptera: Psychidae) with predatory larvae, and eggs individually wrapped in setal cases. Ann Entomol Soc Am 101(4):689–702
de Niceville L (1890) The butterflies of India. Calcutta Central Press Company, Burmah/Ceylon
Dejean A, Beugnon G (1996) Host-ant trail following by myrmecophilous larvae of Liphyrinae (Lepidoptera, Lycaenidae). Oecologia 106(1):57–62
Dejean A, Corbara B, Orivel J, Leponce M (2007) Rainforest canopy ants: the implications of territoriality and predatory behavior. Func Ecosyst Commun 1(2):105–120
Dejean A, Orivel J, Azémar F, Hérault B, Corbara B (2016) A cuckoo-like parasitic moth leads African weaver ant colonies to their ruin. Sci Rep 6(1):1–9
Dejean A, Azémar F, Libert M, Compin A, Herault B, Orivel J, Bouyer T, Corbara B (2017) Ant-lepidopteran associations along African forest edges. Sci Nat 104(1–2):7
Depa Ł, Kaszyca-Taszakowska N, Taszakowski A, Kanturski M (2020) Ant-induced evolutionary patterns in aphids. Biol Rev 95(6):1574–1589
Detrain C, Verheggen FJ, Diez L, Wathelet B, Haubruge E (2010) Aphid–ant mutualism: how honeydew sugars influence the behaviour of ant scouts. Physiol Entomol 35(2):168–174
DeVries PJ (1984) Of crazy-ants and Curetinae: are Curetis butterflies tended by ants? Zool J Linnean Soc 80(1):59–66
DeVries PJ (1988a) The use of epiphylls as larval hostplants by the neotropical riodinid butterfly, Sarota gyas. J Nat Hist 22:1447–1450
DeVries PJ (1988b) The larval ant-organs of Thisbe irenea (Lepidoptera: Riodinidae) and their effects upon attending ants. Zool J Linnean Soc 94(4):379–393
DeVries PJ (1990) Enhancement of symbioses between butterfly caterpillars and ants by vibrational communication. Science 248(4959):1104–1106
DeVries PJ (1991a) Foam barriers, a new defense against ants for milkweed butterfly caterpillars (Nymphalidae: Danainae). J Res Lepidop 30(3–4):261–266
DeVries PJ (1991b) Mutualism between Thisbe irenea butterflies and ants, and the role of ant ecology in the evolution of larval-ant associations. Biol J Linn Soc 43(3):179–195
DeVries PJ (1991c) Ecological and evolutionary patterns in myrmecophilous riodinid butterflies. In: Ant-plant interactions. Oxford University Press, Oxford, pp 143–156
DeVries PJ (1991d) Call production by myrmecophilous riodinid and lycaenid butterfly caterpillars (Lepidoptera): morphological, acoustical, functional, and evolutionary patterns. Am Mus Novit 3025:1–24
DeVries PJ (1997) The butterflies of Costa Rica and their natural history, volume II. Princeton University Press, Riodinidae
DeVries PJ, Baker I (1989) Butterfly exploitation of an ant-plant mutualism: adding insult to herbivory. J N Y Entomol Soc 97(3):332–340
DeVries PJ, Penz CM (2000) Entomophagy, behavior, and elongated thoracic legs in the Myrmecophilous Neotropical butterfly Alesa amesis (Riodinidae). Biotropica 32(4a):712–721
DeVries PJ, Harvey DJ, Kitching IJ (1986) The ant associated epidermal organs on the larva of the lycaenid butterfly Curetis regula Evans. J Nat Hist 20(3):621–633
DeVries PJ, Cabral BC, Penz CM (2004) The early stages of Apodemia paucipuncta (Riodinidae): myrmecophily, a new caterpillar ant-organ and consequences for classification. Milwaukee Pub Mus Contrib 1002:1–13
Deyrup M, Kraus J, Eisner T (2004) A Florida caterpillar and other arthropods inhabiting the webs of a subsocial spider (Lepidoptera: Pyralidae; Araneida: Theridiidae). Fl Entomol 87(4):554–558
Dmitriew CM (2011) The evolution of growth trajectories: what limits growth rate? Biol Rev 86(1):97–116
Dodd FP (1902) Contribution to the life-history of Liphyra brassolis, Westw. Entomologiste 35:153–188
Dodd FP (1912) Some remarkable ant-friend Lepidoptera of Queensland. Trans R Entomol Soc Lond 59(3–4):577–590, plate 48
Dodd FP (1916) Noise-producing Lepidoptera. Etud Lépid compar, Fascicule 11 bis, pp. 13–14
Dolle P, Klein P, Fischer OW, Schnitzler HU, Gilbert LE, Boppré M (2018) Twittering pupae of papilionid and nymphalid butterflies (Lepidoptera): novel structures and sounds. Ann Entomol Soc Am 111(6):341–354
Dookie AL, Young CA, Lamothe G, Schoenle LA, Yack JE (2017) Why do caterpillars whistle at birds? Insect defence sounds startle avian predators. Behav Process 138:58–66
Downes MF, Edwards T (2016) An undescribed concealer moth, ‘Stathmopoda’ sp. (Lepidoptera: Oecophoridae) in nests of the weaver ant ‘Polyrhachis australis’ Mayr (Hymenoptera: Formicidae). Aust Entomol 43(3):161
Downey JC (1966) Sound production in pupae of Lycaenidae. J Lepidop Soc 20:129–155
Downey JC, Allyn AC (1973) Butterfly ultrastructure. 1. Sound production and associated abdominal structures in pupae of Lycaenidae and Riodinidae. Bull Allyn Mus 14:1–47
Downey JC, Allyn AC (1978) Sounds produced in pupae of Lycaenidae. Bull Allyn Mus 48:1–14
Downey JC, Allyn AC (1979) Morphology and biology of the immature stages of Leptotes cassius theonus (Lucas) (Lepid: Lycaenidae). Bull Allyn Mus 55:1–28
Duarte M, Robbins RK (2008) Immature stages of Calycopis bellera (Hewitson) and C. janeirica (Felder) (Lepidoptera, Lycaenidae, Theclinae, Eumaeini): taxonomic significance and new evidence for detritivory. Zootaxa 2325:39–61
Duarte M, Robbins RK (2010) Description and phylogenetic analysis of the Calycopidina (Lepidoptera, Lycaenidae, Theclinae, Eumaeini): a subtribe of detritivores. Rev Bras Entomol 54(1):45–65
Duarte M, Almeida GL, Casagrande MM, Mielke OH (2001) Notes on the last instar larva and pupa of Hemiargus hanno (Stoll) (Lepidoptera, Lycaenidae, Polyommatinae). Rev Bras Zool 18(4):1097–1105. https://doi.org/10.1590/S0101-81752001000400008
Dunn KL (2007) Nocturnal ant attendance of Jalmenus eichhorni Staudinger (Lepidoptera: Lycaenidae) on Cape York Peninsula, Queensland. Calodema 10:35–40
Dunn RR, Agosti D, Andersen AN, Arnan X, Bruhl CA, Cerdá X, Ellison AM, Fisher BL, Fitzpatrick MC, Gibb H, Gotelli NJ (2009) Climatic drivers of hemispheric asymmetry in global patterns of ant species richness. Ecol Lett 12(4):324–333
Dunn RR, Guenard B, Weiser MD, Sanders NJ (2010) Geographic gradients. Ant ecology. Oxford University Press, New York
Dupont S (2012) Structural evolution and diversity of the caterpillar trunk. PhD thesis, University of Copenhagen
Dupont ST, Zemeitat DS, Lohman DJ, Pierce NE (2016) The setae of parasitic Liphyra brassolis butterfly larvae form a flexible armour for resisting attack by their ant hosts (Lycaenidae: Lepidoptera). Biol J Linn Soc 117(3):607–619
Dyer LA (1995) Tasty generalists and nasty specialists? Antipredator mechanisms in tropical lepidopteran larvae. Ecology 76(5):1483–1496
Dyer LA, Singer MS, Lill JT, Stireman JO, Gentry GL, Marquis RJ, Ricklefs RE, Greeney HF, Wagner DL, Morais HC, Diniz IR (2007) Host specificity of Lepidoptera in tropical and temperate forests. Nature 448(7154):696–699
Eastwood R, Fraser AM (1999) Associations between lycaenid butterflies and ants in Australia. Aust J Ecol 24(5):503–537
Eastwood R, Hughes JM (2003) Molecular phylogeny and evolutionary biology of Acrodipsas (Lepidoptera: Lycaenidae). Mol Phylogent Evol 27(1):93–102
Eastwood R, Kitching RL, Manh HB (2005) Behavioral observations on the early stages of Jamides celeno (Cramer) (Lycaenidae) at Cat Tien National Park, Vietnam: an obligate myrmecophile? J Lepidop Soc 59(4):219
Eastwood R, Pierce NE, Kitching RL, Hughes JM (2006) Do ants enhance diversification in lycaenid butterflies? Phylogeographic evidence from a model myrmecophile, Jalmenus evagoras. Ecology 60(2):315–327
Eastwood R, Braby MF, Lohman DJ, King A (2008a) New ant-lycaenid associations and biological data for some Australian butterflies (Lepidoptera: Lycaenidae). Aust Entomol 35(1):47–56
Eastwood R, Braby MF, Schmidt DJ, Hughes JM (2008b) Taxonomy, ecology, genetics and conservation status of the pale imperial hairstreak (Jalmenus eubulus) (Lepidoptera: Lycaenidae): a threatened butterfly from the Brigalow Belt, Australia. Invertebr Syst 22:407–423
Eisner T, Jutro P, Aneshansley DJ, Niedhauk R (1972) Defense against ants in a caterpillar that feeds on ant-guarded scale insects. Ann Entomol Soc Am 65(4):987–988
Ek-Amnuay P (2012) Butterflies of Thailand, 2nd edn. Amarin, Thailand
Ekka PA, Rastogi N (2019) A single lycaenid caterpillar gets an ant-constructed shelter and uninterrupted ant attendance. Entomol Exp Appl 167(12):1012–1019. https://doi.org/10.1111/eea.12859
Ekka PA, Rastogi N, Singh H, Singh HB, Ray S (2020) Lycaenid-tending ants can contribute to fitness gain of the infested host plants by providing nutrients. Arthropod Plant Interact 14(6):745–757
Elfferich NW (1998) Is the larval and imaginal signalling of Lycaenidae and other Lepidoptera related to communication with ants. Deinsea 4:91–95
Elgar MA, Pierce NE (1988) Mating success and fecundity in an ant-tended lycaenid butterfly. Reproductive success. University of Chicago Press, Chicago, pp 59–75
Elgar MA, Nash DR, Pierce NE (2016) Eavesdropping on cooperative communication within an ant-butterfly mutualism. Sci Nat 103(9–10):84
Eliot JN (1973) The higher classification of the Lycaenidae (Lepidoptera): a tentative arrangement. Bull Brit Mus Nat Hist (Entomol) 28:371–505
Eliot JN (1986) A review of the Miletini (Lepidoptera: Lycaenidae). Bull Br Mus Nat Hist 53:1–105
Eliot JN (1990) Notes on the genus Curetis Hubner (Lepidoptera, Lycaenidae). Tyo to Ga 41(4):201–225
Elmes GW, Thomas JA, Munguira ML, Fiedler K (2001) Larvae of lycaenid butterflies that parasitize ant colonies provide exceptions to normal insect growth rules. Biol J Linn Soc 73(3):259–278
Elmes GW, Wardlaw JC, Schönrogge K, Thomas JA (2019) Evidence of a fixed polymorphism of one-year and two-year larval growth in the myrmecophilous butterfly Maculinea rebeli. Insect Conserv Div 12(6):501–510
Eltringham H (1913) The larva of Euliphyra mirifica. Trans Entomol Soc Lond 1913:509–515
Endo S, Itino T (2013) Myrmecophilous aphids produce cuticular hydrocarbons that resemble those of their tending ants. Popul Ecol 55(1):27–34
Entomological Network of Singapore (2017) Homodes larva mimics Oecophylla smaragdina. Available via Facebook. https://www.facebook.com/watch/?v=1938845709677099. Accessed 28 Dec 2020
Epstein ME, Geertsema H, Naumann CM, Tarmann GM (1999) The Zygaenoidea. In: Lepidoptera, moths and butterflies, volume 1: evolution, systematics, and biogeography. Walter de Gruyter, Berlin, pp 159–180
Espeland M, Hall JP, DeVries PJ, Lees DC, Cornwall M, Hsu YF, Wu LW, Campbell DL, Talavera G, Vila R, Salzman S, Ruehr S, Lohman DJ, Pierce NE (2015) Ancient Neotropical origin and recent recolonisation: phylogeny, biogeography and diversification of the Riodinidae (Lepidoptera: Papilionoidea). Mol Phylogent Evol 93:296–306
Espeland M, Breinholt J, Willmott KR, Warren AD, Vila R, Toussaint EF, Maunsell SC, Aduse-Poku K, Talavera G, Eastwood R, Jarzyna MA, Guralnick R, Lohman DJ, Pierce NE, Kawahara AY (2018) A comprehensive and dated phylogenomic analysis of butterflies. Curr Biol 28(5):770–778
Espeland M, Chazot N, Condamine FL, Lemmon A, Lemmon EM, Pringle E, Heath A, Collins S, Tiren W, Nzisa M, Lees D, Fisher S, Woodhall S, Tropek R, Ahlborn SS, Cockburn K, Webb P, Dobson J, Bouyer T, Kaliszewska Z, Baker CM, Talavera G, Vila R, Gardiner A, Williams M, Martins DJ, Safian S, Edge D, Pierce NE (In review) Explosive radiation of ant parasitic butterflies in the genus Lepidochrysops (Lycaenidae) following extinction events during the Miocene aridification of Africa
Eubanks MD, Nesci KA, Petersen MK, Liu Z, Sanchez HB (1997) The exploitation of an ant-defended host plant by a shelter-building herbivore. Oecologia 109(3):454–460
Farquharson CO, Poulton EB, Bagnall RS, Bethune-Baker GT, Chappman TA, Collin JE, Durrant JH, Edwards FW, Eltringham H, Gatenby JB, Newstead R (1922) Five Years' observations (1914–1918) on the bionomics of southern Nigerian insects, chiefly directed to the investigation of Lycaenid life-histories and to the relation of Lycaenidae, Diptera, and other insects to ants. Trans R Entomol Soc Lond 69(3–4):319–531
Faynel C, González-Mercado JH (2019) Crianza de Michaelus phoenissa (Lepidoptera: Lycaenidae) sobre Senna alata (Fabaceae) en Perú. Rev Peru Biol 26(2):265–270. [publication in Spanish]
Fiedler K (1989a) Differences in the behaviour of ants towards two larval instars of Lycaena tityrus (Lep., Lycaenidae). Deut Entomol Zeits 36(4–5):267–271
Fiedler K (1989b) The relationships between lycaenid pupae (Lepidoptera: Lycaenidae) and ants (Hymenoptera: Formicidae). Nachr Ent Ver Apollo Frankfurt 9(1):33–58. [publication in German]
Fiedler K (1991) Systematic, evolutionary, and ecological implications of myrmecophily within the Lycaenidae (Insecta: Lepidoptera: Papilionoidea). Bonn Zool Monogr 31:1–210
Fiedler K (1995) Lycaenid butterflies and plants: is myrmecophily associated with particular hostplant preferences? Ethol Ecol Evol 7(2):107–132
Fiedler K (1998a) Lycaenid-ant interactions of the Maculinea type: tracing their historical roots in a comparative framework. J Insect Conserv 2(1):3–14
Fiedler K (1998b) Geographical patterns in life-history traits of Lycaenidae butterflies—ecological and evolutionary patterns. Zoology 100:336–347
Fiedler K (2001) Ants that associate with Lycaeninae butterfly larvae: diversity, ecology and biogeography. Divers Distrib 7(1–2):45–60
Fiedler K (2006) Ant-associates of Palaearctic lycaenid butterfly larvae (Hymenoptera: Formicidae; Lepidoptera: Lycaenidae)–a review. Myrmecol Nachr 9:77–87
Fiedler K (2012) The host genera of ant-parasitic Lycaenidae butterflies: a review. Psyche A J Entomol 2012:153975
Fiedler K, Hagemann D (1992) The influence of larval age and ant number on myrmecophilous interactions of the African Grass Blue butterfly, Zizeeria knysna (Lepidoptera: Lycaenidae). J Res Lepidop 31:213–232
Fiedler K, Hölldobler B (1992) Ants and Polyommatus icarus immatures (Lycaenidae)—sex-related developmental benefits and costs of ant attendance. Oecologia 91(4):468–473
Fiedler K, Hummel V (1995) Myrmecophily in the brown argus butterfly, Aricia agestis (Lepidoptera: Lycaenidae): effects of larval age, ant number and persistence of contact with ants. Zoology 99:128–137
Fiedler K, Maschwitz U (1988) Functional analysis of the myrmecophilous relationships between ants (Hymenoptera: Formicidae) and lycaenids (Lepidoptera: Lycaenidae). Oecologia 75(2):204–206
Fiedler K, Maschwitz U (1989a) The symbiosis between the weaver ant, Oecophylla smaragdina, and Anthene emolus, an obligate myrmecophilous lycaenid butterfly. J Nat Hist 23(4):833–846
Fiedler K, Maschwitz U (1989b) Adult myrmecophily in butterflies: the role of the ant Anoplolepis longipes in the feeding and oviposition behaviour of Allotinus unicolor (Lepidoptera, Lycaenidae). Tyo to Ga 40(4):241–251
Fiedler K, Saam C (1994) Does ant-attendance influence development in 5 European Lycaenidae butterfly species?(Lepidoptera). Nota Lepidop 17(1/2):5–24
Fiedler K, Seufert P (1995) The mature larva and pupa of Semanga superba (Lepidoptera: Lycaenidae). Nachr Entoml Ver Frankfurt (NF) 16:1–2
Fiedler K, Seufert P, Pierce NE, Pearson JG, Baumgarten HT (1992) Exploitation of lycaenid-ant mutualisms by braconid parasitoids. J Res Lepidop 31:153–168
Fiedler K, Seufert P, Maschwitz U, Azarae I (1995) Notes on larval biology and pupal morphology of Malaysian Curetis butterflies (Lepidoptera: Lycaenidae). Tyo to Ga 45(4):287–299
Fiedler K, Hölldobler B, Seufert P (1996) Butterflies and ants: the communicative domain. Experientia 52(1):14–24
Fletcher LE, Yack JE, Fitzgerald TD, Hoy RR (2006) Vibrational communication in the cherry leaf roller caterpillar Caloptilia serotinella (Gracillarioidea: Gracillariidae). J Insect Behav 19(1):1–8
Floren A, Biun A, Linsenmair EK (2002) Arboreal ants as key predators in tropical lowland rainforest trees. Oecologia 131(1):137–144
Folgarait PJ, Davidson DW (1995) Myrmecophytic Cecropia: antiherbivore defenses under different nutrient treatments. Oecologia 104(2):189–206
Forister ML, Gompert Z, Nice CC, Forister GW, Fordyce JA (2011) Ant association facilitates the evolution of diet breadth in a lycaenid butterfly. Proc R Soc B Biol Sci 278(1711):1539–1547
Forister ML, Novotny V, Panorska AK, Baje L, Basset Y, Butterill PT, Cizek L, Coley PD, Dem F, Diniz IR, Drozd P, Fox M, Glassmire A, Hazen R, Hrcek J, Jahner JP, Kaman O, Kozubowski TJ, Kursar TA, Lewis OT, Lill J, Marquis RJ, Miller SE, Morais HC, Murakami M, Nickel H, Pardikes NA, Ricklefs RE, Singer MS, Smilanich AM, Stireman JO, Villamarin-Cortez S, Vodka S, Volf M, Wagner DL, Walla T, Weiblen GD, Dyer LA (2015) The global distribution of diet breadth in insect herbivores. Proc Natl Acad Sci 112(2):442–447
Franzl S, Locke M, Huie P (1984) Lenticles: innervated secretory structures that are expressed at every other larval moult. Tissue Cell 16:251–268
Fraser AM, Axén AH, Pierce NE (2001) Assessing the quality of different ant species as partners of a myrmecophilous butterfly. Oecologia 129(3):452–460
Fraser AM, Tregenza T, Wedell N, Elgar MA, Pierce NE (2002) Oviposition tests of ant preference in a myrmecophilous butterfly. J Evol Biol 15(5):861–870
Freitas AV (1999) An anti-predator behavior in larvae of Libytheana carinenta (Nymphalidae, Libytheinae). J Lepidop Soc 53:130–131
Freitas AV, Oliveira PS (1996) Ants as selective agents on herbivore biology: effects on the behaviour of a non-myrmecophilous butterfly. J Anim Ecol 65(2):205–210
Fric ZF, Maresova J, Kadlec T, Tropek R, Pyrcz TW, Wiemers M (2019) World travellers: phylogeny and biogeography of the butterfly genus Leptotes (Lepidoptera: Lycaenidae). Syst Entomol 44(3):652–665
Fukuda H, Hama E, Kuzuya T, Taka-hashi A, Takahashi M et al (1984) The life histories of butterflies in Japan, vol III. Hoikusha, Osaka
Funk RS (1975) Association of ants with ovipositing Lycaena rubidus (Lycaenidae). J Lepidop Soc 29:261–262
Fürst MA, Nash DR (2010) Host ant independent oviposition in the parasitic butterfly Maculinea alcon. Biol Lett 6(2):174–176
Geyle HM, Braby MF, Andren M, Beaver EP, Bell P, Bryne C, Castles M, Douglas F, Glatz RV, Haywood B, Hendry P, Kitching RL, Lambkin TA, Meyer CE, Moore MD, Moss JT, Nally S, Rew TR, Palmer CM, Petrie E, Potter-Craven J, Richards K, Sanderson C, Stolarski A, Taylor GS, Williams MR, Woinarski JCZ, Garnett ST (2021) Butterflies on the brink: identifying the Australian butterflies (Lepidoptera) at greatest risk of extinction. Aust Entomol 60:98–110
Gibbs GW (1980) New Zealand butterflies. William Collins Publishers, Auckland
Glasier JR, Poore AG, Eldridge DJ (2018) Do mutualistic associations have broader host ranges than neutral or antagonistic associations? A test using myrmecophiles as model organisms. Insect Soc 65(4):639–648
Gnatzy W, Jatho M, Kleinteich T, Gorb SN, Hustert R (2017) The eversible tentacle organs of Polyommatus caterpillars (Lepidoptera, Lycaenidae): morphology, fine structure, sensory supply and functional aspects. Earth Struct Dev 46(6):788–804
Goitía W, Jaffé K (2009) Ant-plant associations in different forests in Venezuela. Neotrop Entomol 38(1):7–31
Gompert Z, Fordyce JA, Forister ML, Nice CC (2008) Recent colonization and radiation of north American Lycaeides (Plebejus) inferred from mtDNA. Mol Phylogent Evol 48:481–490
Gotthard K (2000) Increased risk of predation as a cost of high growth rate: an experimental test in a butterfly. J Anim Ecol 69(5):896–902
Gray B (1974) Associated fauna found in nests of Myrmecia (hymenoptera: Formicidae). Insect Soc 21(3):289–299
Grover CD, Kay AD, Monson JA, Marsh TC, Holway DA (2007) Linking nutrition and behavioural dominance: carbohydrate scarcity limits aggression and activity in argentine ants. Proc R Soc B Biol Sci 274(1628):2951–2957
Hall JP (1998) A review of the genus Sarota (Lepidoptera: Riodinidae). Trop Lepidop 9(supplement 1):1–21
Hall JP (2007) Phylogenetic revision of the new neotropical riodinid genus Minstrellus (Lepidoptera: Riodinidae). Ann Entomol Soc Am 100(6):773–786
Hall JP (2018) A monograph of the Nymphidiina (Lepidoptera: Riodinidae: Nymphidiini): phylo-geny, taxonomy, biology, and biogeography. Entomological Society of Washington, Washington, DC
Hall JP, Harvey DJ (2001) A phylogenetic analysis of the Neotropical riodinid butterfly genera Juditha, Lemonias, Thisbe and Uraneis, with a revision of Juditha (Lepidoptera: Riodinidae: Nymphidiini). Syst Entomol 26(4):453–490
Hall JP, Harvey DJ (2002) Basal subtribes of the Nymphidiini (Lepidoptera: Riodinidae): phylogeny and myrmecophily. Cladistics 18(6):539–569
Hall JP, Harvey DJ, Janzen DH (2004) Life history of Calydna sturnula with a review of larval and pupal balloon setae in the Riodinidae (Lepidoptera). Ann Entomol Soc Am 97(2):310–321
Hardy PB, Lawrence JB (2017) Field guide to butterflies of the Philippines. Siri Scientific Press
Harvey DJ, Longino J (1989) Myrmecophily and larval food plants of Brephidium isophthalma pseudofea (Lycaenidae) in the Florida keys. J Lepidop Soc 43(4):332–333
Harvey DJ, Webb TA (1980) Ants associated with Harkenclenus titus, Glaucopsyche lygdamus, and Celastrina argiolus (Lycaenidae). J Lepidop Soc 34(4):371–372
Hawkeswood TJ, Dunn KL, Sommung B (2016) Acanthus ilicifolius L.(Acanthaceae), a new larval host plant for Hypolycaena erylus himavantus Fruhstorfer, 1912 (Lepidoptera: Lycaenidae) from Thailand, with a review on larval host plants of H. erylus and its weaver ant association. Calodema 416:1–8
Heath A (1997) A review of African genera of the tribe Aphnaeini (Lepidoptera: Lycaenidae). Metamorphosis Occ Suppl 2:1–60
Heath A, Claassens AJ (2000) New observations of ant associations and life history adaptations (Lepidoptera: Lycaenidae) in South Africa. Metamorphosis 11:3–19
Heath A, Claassens AJ (2003) Ant-association among southern African Lycaenidae. J Lepidop Soc 57(1):1–16
Heath A, Pringle EL (2004) A review of the southern African genus Thestor Hübner (Lepidoptera: Lycaenidae: Miletinae). Metamorphosis 15:89–151
Heikkilä M, Mutanen M, Wahlberg N, Sihvonen P, Kaila L (2015) Elusive ditrysian phylogeny: an account of combining systematized morphology with molecular data (Lepidoptera). BMC Evol Biol 15(1):260
Heil M, Hilpert A, Fiala B, Linsenmair K (2001) Nutrient availability and indirect (biotic) defence in a Malaysian ant-plant. Oecologia 126(3):404–408
Heil M, Barajas-Barron A, Orona-Tamayo D, Wielsch N, Svatos A (2014) Partner manipulation stabilises a horizontally transmitted mutualism. Ecol Lett 17(2):185–192
Henning SF (1983) Chemical communication between lycaenid larvae (Lepidoptera: Lycaenidae) and ants (Hymenoptera: Formicidae). J Entomol Soc S Afr 46(2):341–366
Heredia MD, Robbins RK (2016) Natural history of the mistletoe-feeding Thereus lomalarga (Lepidoptera, Lycaenidae, Eumaeini) in Colombia. Zootaxa 4117(3):301–320
Hill CJ (1993) The myrmecophilous organs of Arhopala madytus Fruhstorfer (Lepidoptera: Lycaenidae). J Aust Entomol Soc 32:283–288
Hinton HE (1951) Myrmecophilous Lycaenidae and other Lepidoptera—a summary. Proc Lond Entomol Nat Hist Soc 1949–1950:111–175
Hocking B (1970) Insect associations with the swollen thorn acacias. Trans R Entomol Soc Lond 122(pt. 7):211–255
Hojo MK, Wada-Katsumata A, Akino T, Yamaguchi S, Ozaki M, Yamaoka R (2009) Chemical disguise as particular caste of host ants in the ant inquiline parasite Niphanda fusca (Lepidoptera: Lycaenidae). Proc R Soc B Biol Sci 276(1656):551–558
Hojo MK, Wada-Katsumata A, Ozaki M, Yamaguchi S, Yamaoka R (2008) Gustatory synergism in ants mediates a species-specific symbiosis with lycaenid butterflies. J Comput Physiol A Neuroethol Sens Neural Behav Physiol 194(12):1043–1052
Hojo MK, Yamaguchi S, Akino T, Yamaoka R (2014a) Adoption of lycaenid Niphanda fusca (Lepidoptera: Lycaenidae) caterpillars by the host ant Camponotus japonicus (Hymenoptera: Formicidae). Entomol Sci 17(1):59–65
Hojo MK, Yamamoto A, Akino T, Tsuji K, Yamaoka R (2014b) Ants use partner specific odors to learn to recognize a mutualistic partner. PLoS One 9(1):e86054
Hojo MK, Pierce NE, Tsuji K (2015) Lycaenid caterpillar secretions manipulate attendant ant behavior. Curr Biol 25(17):2260–2264
Hölldobler B, Kwapich C (in review) The guests of ants: how myrmecophiles interact with their hosts
Hölldobler B, Wilson EO (1990) The ants. Harvard University Press, Cambridge. https://www.antwiki.org/wiki/The_Ants
Holloway JD (2005) The moths of Borneo parts 15 & 16: Family Noctuidae, Subfamily Catocalinae. Southdene Sdn Bhd. Available at https://www.mothsofborneo.com/part-15-16/index.htm. Accessed 3 Jan 2020
Honda K (1983) Defensive potential of components of the larval osmeterial secretion of papilionid butterflies against ants. Physiol Entomol 8(2):173–179
Hopper SD (2009) OCBIL theory: towards an integrated under- standing of the evolution, ecology and conservation of biodiversity on old, climatically buffered, infertile landscapes. Plant Soil 322:49–86
Horvitz CC, Turnbull C, Harvey DJ (1987) Biology of immature Eurybia elvina (Lepidoptera: Riodinidae), a myrmecophilous metalmark butterfly. Ann Entomol Soc Am 80(4):513–519
Hsu YF, Johnson KU (1998) A New species of Zinaspa from China (Lepidoptera: Lycaenidae: Theclinae). Holarct Lepidop 5(2):39–42
Hsu YF, Ding D, Yen SH, Qian ZQ (2004) Systematic problems surrounding Howarthia melli (Forster)(Lepidoptera: Lycaenidae: Theclinae), with description of a new species and a review of Rhododendron-association in lycaenid butterflies. Ann Entomol Soc Am 97(4):653–666
Igarashi S, Fukuda H (1997) The life histories of Asian butterflies, vol 1. Tokai University Press, Tokyo
Igarashi S, Fukuda H (2000) The life histories of Asian butterflies, vol 2. Tokai University Press, Tokyo
Inui Y, Shimizu-kaya U, Okubo T, Yamsaki E, Itioka T (2015) Various chemical strategies to deceive ants in three Arhopala species (Lepidoptera: Lycaenidae) exploiting Macaranga myrmecophytes. PLoS One 10(4):e0120652
Itioka T, Yamamoto T, Tzuchiya T, Okubo T, Yago M, Seki Y, Ohshima Y, Katsuyama R, Chiba H, Yata O (2009) Butterflies collected in and around Lambir Hills National Park, Sarawak, Malaysia in Borneo. Contrib Biol Lab Kyoto Univ 30(1):25–68
Ito F, Higashi S (1991) Variance of ant effects on the different life forms of moth caterpillars. J Anim Ecol 1:327–334
IUCN (2020) The IUCN Red list of threatened species. https://www.iucnredlist.org
Ivens AB (2015) Cooperation and conflict in ant (Hymenoptera: Formicidae) farming mutualisms: a review. Myrmecol News 21:19–36
Ivens AB, von Beeren C, Blüthgen N, Kronauer DJ (2016) Studying the complex communities of ants and their symbionts using ecological network analysis. Annu Rev Entomol 61:353–371
Jackson TH (1937) The early stages of some African Lycaenidae (Lepidoptera), with an account of the larval habits. Trans R Entomol Soc Lond 86(12):201–237
Jackson TH (1957) Protective mimicry in the pupa of Epitola urania (Lep.: Lycaenidae). J Entomol Soc S Afr 20(2):234
Janzen DH (1967) Interaction of the bull's-horn acacia (Acacia cornigera L.) with an ant inhabitant (Pseudomyrmex ferrugineus F. Smith) in eastern Mexico. Kansas Univ Sci Bull 47:315–558
Janzen DH (1984) Two ways to be a tropical big moth: Santa Rosa saturniids and sphingids. Oxf Surv Evol Biol 1:85–146
Janzen DH, Hallwachs W (2021) Dynamic database for an inventory of the macrocaterpillar fauna, and its food plants and parasitoids, of Area de Conservacion Guanacaste (ACG), northwestern Costa Rica. http://janzen.sas.upenn.edu. Accessed March 2021
Jeratthitikul E, Yago M, Shizuya H, Yokoyama J, Hikida T (2011) Life history and morphology of the black cupid butterfly, Tongeia kala (De Nicéville)(Lycaenidae), from Myanmar. J Lepidop Soc 65(3):167–174
Johnson SJ, Valentine PS (2001) The life history of Nesolycaena medicea Braby (Lepidoptera: Lycaenidae). Aust Entomol 27(4):109–112
Jones MT, Castellanos I, Weiss MR (2002) Do leaf shelters always protect caterpillars from invertebrate predators? Ecol Entomol 27(6):753–757
Kaliszewska ZA, Lohman DJ, Sommer K, Adelson G, Rand DB, Mathew J, Talavera G, Pierce NE (2015) When caterpillars attack: biogeography and life history evolution of the Miletinae (Lepidoptera: Lycaenidae). Evolution 69(3):571–588
Kaminski LA (2008a) Immature stages of Caria plutargus (Lepidoptera: Riodinidae), with discussion on the behavioral and morphological defensive traits in nonmyrmecophilous riodinid butterflies. Ann Entomol Soc Am 101(5):906–914
Kaminski LA (2008b) Polyphagy and obligate myrmecophily in the butterfly Hallonympha paucipuncta (Lepidoptera: Riodinidae) in the Neotropical Cerrado savanna. Biotropica 40(3):390–394
Kaminski LA (2017) In: Diehl E (ed) Formigas, besouros e lepidópteros. Interações das formigas com outros organismos: diversidade ecológica e evolutiva. Oikos, São Leopoldo, pp 51–65
Kaminski LA, Carvalho-Filho FS (2012) Life history of Aricoris propitia (Lepidoptera: Riodinidae)—a myrmecophilous butterfly obligately associated with fire ants. Psyche 2012:1–10
Kaminski LA, Rodrigues D (2011) Species-specific levels of ant attendance mediate performance costs in a facultative myrmecophilous butterfly. Physiol Entomol 36(3):208–214
Kaminski LA, Freitas AV, Oliveira PS (2010a) Interaction between mutualisms: ant-tended butterflies exploit enemy-free space provided by ant-treehopper associations. Am Nat 176(3):322–334
Kaminski LA, Thiele SC, Iserhard CA, Romanowski HP, Moser A (2010b) Natural history, new records, and notes on the conservation status of Cyanophrys bertha (Jones) (Lepidoptera: Lycaenidae). Proc Entomol Soc Wash 112(1):54–60
Kaminski LA, Mota LL, Freitas AV (2012a) Life history of Porthecla ravus (Druce) (Lepidoptera: Lycaenidae), with discussion on the use of Annonaceae by Eumaeini butterflies. Annales de la Société entomologique de France 48(3–4):309–312
Kaminski LA, Rodrigues D, Freitas AV (2012b) Immature stages of Parrhasius polibetes (Lepidoptera: Lycaenidae): host plants, tending ants, natural enemies and morphology. J Nat Hist 46(11–12):645–667
Kaminski LA, Mota LL, Freitas AV, Moreira GR (2013) Two ways to be a myrmecophilous butterfly: natural history and comparative immature-stage morphology of two species of Theope (Lepidoptera: Riodinidae). Biol J Linn Soc 108(4):844–870
Kaminski LA, Mota LL, Freitas AV (2014) Larval cryptic coloration and mistletoe use in the metalmark butterfly Dachetola azora (Lepidoptera: Riodinidae). Entomol Am 120(1):18–23
Kaminski LA, Volkmann L, Ríos S, Vila R (2015) Ecology and evolution of Aricoris chilensis complex (Riodinidae): integrative taxonomy reveal cryptic species. En Lepid Neotrop
Kaminski LA, Iserhard CA, Freitas AV (2016) Thisbe silvestre sp. nov. (Lepidoptera: R iodinidae): a new myrmecophilous butterfly from the Brazilian Atlantic Forest. Aust Entomol 55(2):138–146
Kaminski LA, Carneiro E, Dolibaina DR, Casagrande MM, Mielke OH (2020a) Oviposition of Minstrellus grandis (Lepidoptera: Riodinidae) in a harmful ant-plant symbiosis. Acta Amazon 50(3):256–259
Kaminski LA, Volkmann L, Callaghan CJ, DeVries PJ, Vila R (2020b) The first known riodinid ‘cuckoo’ butterfly reveals deep-time convergence and parallelism in ant social parasites. Zool J Linnean Soc 192:1–20
Kaspari M (2020) The seventh macronutrient: how sodium shortfall ramifies through populations, food webs and ecosystems. Ecol Lett 23:1153–1168. https://doi.org/10.1111/ele.13517
Kaspari M, Vargo EL (1995) Colony size as a buffer against seasonality: Bergmann's rule in social insects. Am Nat 145(4):610–632
Kaspari M, Alonso L, O’Donnell S (2000) Three energy variables predict ant abundance at a geographical scale. Proc R Soc Lond Ser B Biol Sci 267(1442):485–489
Kaspari M, Yanoviak SP, Dudley R (2008) On the biogeography of salt limitation: a study of ant communities. Proc Natl Acad Sci 105(46):17848–17851
Kaspari M, Yanoviak SP, Dudley R, Yuan M, Clay NA (2009) Sodium shortage as a constraint on the carbon cycle in an inland tropical rainforest. Proc Natl Acad Sci 106(46):19405–19409
Kaspari M, Donoso D, Lucas JA, Zumbusch T, Kay AD (2012) Using nutritional ecology to predict community structure: a field test in Neotropical ants. Ecosphere 3(11):1–5
Kaspari M, Welti EA, de Beurs KM (2020) The nutritional geography of ants: gradients of sodium and sugar limitation across North American grasslands. J Anim Ecol 89(2):276–284
Kawahara AY, Plotkin D, Espeland M, Meusemann K, Toussaint EF, Donath A, Gimnich F, Frandsen PB, Zwick A, dos Reis M, Barber JR, Peters RS, Liu S, Zhou X, Mayer C, Podsiadlowski L, Storer C, Yack JE, Misof B, Breinholt JW (2019) Phylogenomics reveals the evolutionary timing and pattern of butterflies and moths. Proc Natl Acad Sci 116(45):22657–22663
Kim SS, Ho YH (2012) Life histories of Korean butterflies. Sagyejel, Paju [publication in Korean]
Kistner DH (1982) The social insects’ bestiary. In: Social insects. Academic, New York, pp 1–244
Kitching RL (1983) Myrmecophilous organs of the larvae and pupa of the lycaenid butterfly Jalmenus evagoras (Donovan). J Nat Hist 17(3):471–481
Kitching RL, Luke B (1985) The myrmecophilous organs of the larvae of some British Lycaenidae (Lepidoptera): a comparative study. J Nat Hist 19(2):259–276
Koh LP, Dunn RR, Sodhi NS, Colwell RK, Proctor HC, Smith VS (2004) Species coextinctions and the biodiversity crisis. Science 305(5690):1632–1634
Komatsu T, Itino T (2014) Moth caterpillar solicits for homopteran honeydew. Nat Sci Rep 4(1):1–3
Koptur S (1985) Alternative defenses against herbivores in Inga (Fabaceae: Mimosoideae) over an elevational gradient. Ecology 66(5):1639–1650
Kronauer DJ, Pierce NE (2011) Myrmecophiles. Curr Biol 21(6):R208–R209
Kubik TD, Schorr RA (2018) Facultative myrmecophily (Hymenoptera: Formicidae) in the hops blue butterfly, Celastrina humulus (Lepidoptera: Lycaenidae). Entomol News 127(5):490–498
Kumar KP, Kamala Jayanthi PD, Verghese A, Chakravarthy AK (2017) Facultative myrmecophily in Deudorix isocrates (Fabricius) (Lepidoptera: Lycaenidae). J Entomol Zool Stud 5(5):870–875
Lafranchis T (2019) Notes on the biology of some butterflies in Greece (Lepidoptera: Papilionoidea). Entomol Gaz 70(2):113–134
Lafranchis T, Gil-T FE, Lafranchis A (2007) New data on the ecology of 8 taxa of Agrodiaetus Hubner, 1822 from Greece and Spain: hostplants, associated ants and parasitoids. Atalanta 38(1/2):189–197
Lamas G, Callaghan C, Casagrande MM, Mielke O, Pyrcz T, Robbins R, and Viloria A (2004) Hesperioidea – Papilionoidea. Association for Tropical Lepidoptera/Scientific Publishers, Gainesville. Digitized by Symons F at https://www.ucl.ac.uk/taxome/gbn/
Lamborn WA (1911) Some ant-tended lycaenid larvae observed by Mr. W. A. Lamborn in the Lagos District. Proc Entomol Soc Lond 1911:xcix–cvii
Lamborn WA, Bethune-Baker GT, Distant WL, Eltringham H, Poulton EB, Durrant JH, Newstead R (1914) XX. On the relationship between certain west African insects, especially ants, Lycaenidae and Homoptera. Trans R Entomol Soc Lond 61(3):436–498
Larsen TB (2005) Butterflies of West Africa. Apollo Books, Stenstrup
Léger T, Mally R, Neinhuis C, Nuss M (2021) Refining the phylogeny of Crambidae with complete sampling of subfamilies (Lepidoptera, Pyraloidea). Zool Scr 50(1):84–99
Leimar O, Axén AH (1993) Strategic behaviour in an interspecific mutualism: interactions between lycaenid larvae and ants. Anim Behav 46(6):1177–1182
Lengyel S, Gove AD, Latimer AM, Majer JD, Dunn RR (2010) Convergent evolution of seed dispersal by ants, and phylogeny and biogeography in flowering plants: a global survey. Perspect Plant Ecol Evol Syst 12(1):43–55
Leong TM, D’Rozario V (2012) Mimicry of the weaver ant, Oecophylla smaragdina by the moth caterpillar, Homodes bracteigutta, the crab spider, Amyciaea lineatipes, and the jumping spider, Myrmarachne plataleoides. Nat Singapore 5:39–56
Lima LD, Trigo JR, Kaminski LA (2020) Chemical convergence between a guild of facultative myrmecophilous caterpillars and host plants. Ecol Entomol. https://doi.org/10.1111/een.12941
Lin YH, Liao YC, Yang CC, Billen J, Yang MM, Hsu YF (2019) Vibrational communication between a myrmecophilous butterfly Spindasis lohita (Lepidoptera: Lycaenidae) and its host ant Crematogaster rogenhoferi (Hymenoptera: Formicidae). Sci Rep 9(1):1
Linsley E (1944) Natural sources, habitats, and reservoirs of insects associated with stored food products. Hilgardia 16(4):185–224
Lo YF, Li F, Ding L (2017) Description of a new subspecies of Talicada nyseus (Guérin-Méneville, 1843) from Hainan, China (Lepidoptera: Lycaenidae), with notes on the genus Talicada Moore, 1881. Zootaxa 4269(4):586–592
Loeffler CC (1996) Caterpillar leaf folding as a defense against predation and dislodgment: staged encounters using Dichomeris (Gelechiidae) larvae on goldenrods. J Lepidop Soc 50(3):245–260
Lohman DJ (2004) Cuticular hydrocarbons and chemical camouflage in butterfly-ant symbioses (Lepidoptera: Lycaenidae; Hymenoptera: Formicidae). PhD Thesis, Harvard University, Cambridge
Lohman DJ, Samarita VU (2009) The biology of carnivorous butterfly larvae (Lepidoptera: Lycaenidae: Miletinae: Miletini) and their ant-tended hemipteran prey in Thailand and the Philippines. J Nat Hist 43(9–10):569–581
Lohman DJ, Liao Q, Pierce NE (2006) Convergence of chemical mimicry in a guild of aphid predators. Ecol Entomol 31(1):41–51
Lokkers C (1990) Colony dynamics of the green tree ant (Oecophylla smaragdina Fab.) in a seasonal tropical climate. PhD Thesis, James Cook University, Queensland
Madeiros LP, Garcia G, Thompson JN, Guimarães PR (2018) The geographic mosaic of coevolution in mutualistic networks. Proc Natl Acad Sci U S A 115:12017–12022
Malicky H (1969) Versuch einer Analyse der okologischen Beziehungen zwischen Lycaeniden (Lepidoptera) und Formiciden (Hymenoptera). Tijd Entomol 112:213–298. (publication in German)
Malicky H (1970) New aspects of the association between lycaenid larvae (Lycaenidae) and ants (Formicidae, Hymenoptera). J Lepidop Soc 24(3):190–202
Mally R, Hayden JE, Neinhuis C, Jordal BH, Nuss M (2019) The phylogenetic systematics of Spilomelinae and Pyraustinae (Lepidoptera: Pyraloidea: Crambidae) inferred from DNA and morphology. Earth Syst Phylogent 77(1):141–204
Mänd T, Tammaru T, Mappes J (2007) Size dependent predation risk in cryptic and conspicuous insects. Evol Ecol 21(4):485–498
Martins DJ, Collins SC, Congdon C, Pierce NE (2013) Association between the African lycaenid, Anthene usamba, and an obligate acacia ant, Crematogaster mimosae. Biol J Linn Soc 109(2):302–312
Maschwitz U, Schroth M, Hänel H, Pong TY (1984) Lycaenids parasitizing symbiotic plant-ant partnerships. Oecologia 64(1):78–80
Maschwitz U, Dumpert K, Tuck KR (1986) Ants feeding on anal exudate from tortricid larvae: a new type of trophobiosis. J Nat Hist 20(5):1041–1050
Maschwitz U, Nassig WA, Dumpert K, Fiedler K (1988) Larval carnivory and myrmecoxeny, and imaginal myrmecophily in miletine lycaenids (Lepidoptera, Lycaenidae) on the Malay peninsula. Tyo to Ga 39(3):167–181
Mathew J, Travassos MA, Canfield MR, Murawski DA, Kitching RL, Pierce NE (2008) The singing reaper: diet, morphology and vibrational signaling in the nearctic species Feniseca tarquinius (Lepidoptera: Lycaenidae, Miletinae). Trop Lepidop Res 1:24–29
Meehan CJ, Olson EJ, Reudink MW, Kyser TK, Curry RL (2009) Herbivory in a spider through exploitation of an ant–plant mutualism. Curr Biol 19(19):R892–R893
Megens HJ, De Jong R, Fiedler K (2005) Phylogenetic patterns in larval host plant and ant association of indo-Australian Arhopalini butterflies (Lycaenidae: Theclinae). Biol J Linn Soc 84(2):225–241
Merrill, DN (1997) Deception in lycaenid-ant mutualism? Senior thesis, Harvard University, Cambridge
Michelangeli FA (2003) Ant protection against herbivory in three species of Tococa (Melastomataceae) occupying different environments 1. Biotropica 35(2):181–188
Miller CG, Lane DA (2004) A new species of Acrodipsas Sands (Lepidoptera: Lycaenidae) from the Northern Territory. Aust Entomol 31:141–146
Mitter C, Davis DR, Cummings MP (2017) Phylogeny and evolution of Lepidoptera. Annu Rev Entomol 62:265–283
Mizuno T, Hagiwara Y, Akino T (2018) Chemical tactic of facultative myrmecophilous lycaenid pupa to suppress ant aggression. Chemoecology 28(6):173–182
Mizuno T, Hagiwara Y, Akino T (2019) Varied effects of tending ant species on the development of facultatively myrmecophilous lycaenid butterfly larvae. Insects 10(8):234
Morozumi Y, Murakami T, Watanabe M, Ohta S, Ômura H (2019) Absence of cuticular alkenes allows lycaenid larvae to avoid predation by Formica japonica ants. Entomol Sci 22(2):126–136
Mota LL, Oliveira PS (2016) Myrmecophilous butterflies utilise ant–treehopper associations as visual cues for oviposition. Ecol Entomol 41:338–343
Mota LL, Kaminski LA, Freitas AV (2014) Last instar larvae and pupae of Ourocnemis archytas and Anteros formosus (Lepidoptera: Riodinidae), with a summary of known host plants for the tribe Helicopini. Zootaxa 3838(4):435–444
Mota LL, Kaminski LA, Freitas AV (2020) The tortoise caterpillar: carnivory and armoured larval morphology of the metalmark butterfly Pachythone xanthe (Lepidoptera: Riodinidae). J Nat Hist 54(5–6):309–319
Musche M, Anton C, Worgan A, Settele J (2006) No experimental evidence for host ant related oviposition in a parasitic butterfly. J Insect Behav 19(5):631–643
Nash DR (1990) Cost-benefit analysis of a mutualism between lycaenid butterflies and ants. PhD thesis, Oxford University
Nash DR, Als TD, Maile R, Jones GR, Boomsma JJ (2008) A mosaic of chemical coevolution in a large blue butterfly. Science 319(5859):88–90
Neild AF, Bálint Z (2014) Notes on the identity of Evenus coronata (Hewitson, 1865) (Lepidoptera: Lycaenidae: Theclinae: Eumaeini) with the description of a remarkably overlooked sibling species. Trop Lepidop Res 24(2):105–120
New TR (ed) (1993) Conservation biology of Lycaenidae (butterflies). IUCN, Gland
Newcomer EJ (1912) Some observations on the relations of ants and lycaenid caterpillars, and a description of the relational organs of the latter. J N Y Entomol Soc 20(1):31–36
Nichols SW (1989) Torre-Bueno glossary of entomology. Entomological Society, New York
Nielsen GJ, Kaminski LA (2018) Immature stages of the Rubiaceae-feeding metalmark butterflies (Lepidoptera: Riodinidae), and a new function for the tentacle nectary organs. Zootaxa 4524(1):1–32
Nishida K (2010) Description of the immature stages and life history of Euselasia (Lepidoptera: Riodinidae) on Miconia (Melastomataceae) in Costa Rica. Zootaxa 2466:1–74
Nishida K, Robbins RK (2020) One side makes you taller: a mushroom–eating butterfly caterpillar (Lycaenidae) in Costa Rica. Neotrop Biol Conserv 15(4):463–470
Nyffeler M, Olson EJ, Symondson WO (2016) Plant-eating by spiders. J Arachnol 44:15–27
Obregón R, Shaw MR, Fernández-Haeger J, Jordano D (2015) Parasitoid and ant interactions of some Iberian butterflies (Insecta: Lepidoptera). SHILAP Rev Lepidopterol 43(171):439–454
Offenberg J (2001) Balancing between mutualism and exploitation: the symbiotic interaction between Lasius ants and aphids. Behav Ecol Sociobol 49(4):304–310
Okubo T, Yago M, Itioka T (2009) Immature stages and biology of Bornean Arhopala butterflies (Lepidoptera, Lycaenidae) feeding on myrmecophytic Macaranga. Trans Lepidop Soc Jpn 60(1):37–51
Oliver JC (2007) Population, phylogenetic, and coalescent analyses of character evolution in gossamer-winged butterflies (Lepidoptera: Lycaenidae). Dissertation, University of Arizona
Omura H, Watanabe M, Honda K (2009) Cuticular hydrocarbons of larva and pupa of Reverdin's blue, Lycaeides argyrognomon (Lycaenidae) and its tending ants. Trans Lepidop Soc Jpn 60(3):203–210
Opler PA (1992) A field guide to eastern butterflies. Houghton Mifflin Co, New York
Opler PA (1999) A field guide to western butterflies. Houghton Mifflin Co, New York
Orivel J, Dejean A (2000) Myrmecophily in Hesperiidae. The case of Vettius tertianus in ant gardens. Comptes Rend l’Académ Sci-Ser III-Sci Vie 323(8):705–715
Orivel J, Servigne P, Cerdan P, Dejean A, Corbara B (2004) The ladybird Thalassa saginata, an obligatory myrmecophile of Dolichoderus bidens ant colonies. Naturwissenschaften 91(2):97–100
Orr AG, Charles JK, Yahya HH, Sharebini NH (1996) Nesting and colony structure in the giant forest ant, Camponotus gigas (Latreille) (Hymenoptera: Formicidae). Raffles Bull Zool 44:247–252
Painting C, Nicholson CC, Bulbert MW, Norma-Rashid Y, Li D (2017) Nectary feeding and guarding behavior by a tropical jumping spider. Front Ecol Environ 15(8):469–470
Palting JD (2020) A Molecular Phylogenetic Assessment of the North American Lichen Tiger Moths (Lepidoptera; Erebidae; Arctiinae; Lithosiini) with Life History Observations and a Description of a New Species from Central Arizona. Dissertation, University of Arizona
Pan TS, Morishita K (1990) Notes on Nacaduba normani Eliot in Sabah, North Borneo (Lepidoptera, Lycaenidae). Tyo to Ga 41(3):149–154
Parker J (2016) Myrmecophily in beetles (Coleoptera): evolutionary patterns and biological mechanisms. Myrmecol News 22:65–108
Parmentier T, Dekoninck W, Wenseleers T (2014) A highly diverse microcosm in a hostile world: a review on the associates of red wood ants (Formica rufa group). Insect Soc 61(3):229–237
Parsons M (1984) Life histories of four species of Philiris Roeber (Lepidoptera: Lycaenidae) from Papua New Guinea. J Lepidop Soc 38(1):15–22
Parsons M (1999) The butterflies of Papua New Guinea: their systematics and biology. Academic, Cambridge
Patricelli D, Barbero F, Occhipinti A, Bertea CM, Bonelli S, Casacci LP, Zebelo SA, Crocoll C, Gershenzon J, Maffei ME, Thomas JA (2015) Plant defences against ants provide a pathway to social parasitism in butterflies. Proc R Soc B Biol Sci 282(1811):20151111
Patricelli D, Barbero F, La Morgia V, Casacci LP, Witek M, Balletto E, Bonelli S (2011) To lay or not to lay: oviposition of Maculinea arion in relation to Myrmica ant presence and host plant phenology. Anim Behav 82(4):791–799
Paul RG (1977) Aspects of the biology and taxonomy of British myrmecophilous root aphids. PhD thesis, University of London, London
Pech P, Fric Z, Konvicka M (2007) Species-specificity of the Phengaris (Maculinea)–Myrmica host system: fact or myth?(Lepidoptera: Lycaenidae; Hymenoptera: Formicidae). Sociobiology 50(3):983–1004
Pellissier L, Litsios G, Guisan A, Alvarez N (2012) Molecular substitution rate increases in myrmecophilous lycaenid butterflies (Lepidoptera). Zool Scr 41(6):651–658
Pellissier L, Litsios G, Fiedler K, Pottier J, Dubuis A, Pradervand JN, Salamin N, Guisan A (2012a) Loss of interactions with ants under cold climate in a regional myrmecophilous butterfly fauna. J Biogeogr 39(10):1782–1790
Pellissier L, Rasmann S, Litsios G, Fiedler K, Dubuis A, Pottier J, Guisan A (2012b) High host-plant nitrogen content: a prerequisite for the evolution of ant–caterpillar mutualism? J Evol Biol 25(8):1658–1666
Penz CM, DeVries PJ (2006) Systematic position of Apodemia paucipuncta (Riodinidae), and a critical evaluation of the nymphidiine transtilla. Zootaxa 1190(1):1–50
Peterson MA (1993) The nature of ant attendance and the survival of larval Icaricia acmon (Lycaenidae). J Lepidop Soc 47(1):8–16
Peterson SC, Johnson ND, LeGuyader JL (1987) Defensive regurgitation of allelochemicals derived from host cyanogenesis by eastern tent caterpillars. Ecology 68(5):1268–1272
Pierce NE (1984) Amplified species diversity: a case study of an Australian lycaenid butterfly and its attendant ants. In: The biology of butterflies. Academic, London, pp 197–200
Pierce NE (1985) Lycaenid butterflies and ants: selection for nitrogen fixing and other protein rich food plants. Am Nat 125:888–895
Pierce NE (1987) The evolution and biogeography of associations between lycaenid butterflies and ants. Oxf Surv Evol Biol 4:89–116
Pierce NE (1995) Predatory and parasitic Lepidoptera: carnivores living on plants. J Lepidop Soc 49(4):412–453
Pierce NE, Easteal S (1986) The selective advantage of attendant ants for the larvae of a lycaenid butterfly, Glaucopsyche lygdamus. J Anim Ecol 55(2):451–462
Pierce NE, Elgar MA (1985) The influence of ants on host plant selection by Jalmenus evagoras, a myrmecophilous lycaenid butterfly. Behav Ecol Sociobiol 3:209–222
Pierce NE, Mead PS (1981) Parasitoids as selective agents in the symbiosis between lycaenid butterfly larvae and ants. Science 211(4487):1185–1187
Pierce NE, Nash DR (1999) The imperial blue, Jalmenus evagoras (Lycaenidae). Mon Aust Lepidop 6:277–313
Pierce NE, Kitching RL, Buckley RC, Taylor MF, Benbow KF (1987) The costs and benefits of cooperation between the Australian lycaenid butterfly, Jalmenus evagoras, and its attendant ants. Behav Ecol Sociobiol 21(4):237–248
Pierce NE, Braby MF, Heath A, Lohman DJ, Mathew J, Rand DB, Travassos MA (2002) The ecology and evolution of ant association in the Lycaenidae (Lepidoptera). Annu Rev Entomol 47(1):733–771
Pohl S, Frederickson ME, Elgar MA, Pierce NE (2016) Colony diet influences ant worker foraging and attendance of myrmecophilous lycaenid caterpillars. Front Ecol Evol 4:114
Portugal AHA, Trigo JR (2005) Similarity of cuticular lipids between a caterpillar and its host plant: a way to make prey undetectable for predatory ants? J Chem Ecol 31(11):2551–2561
Poulton EB (1890) The colours of animals, their meaning and use, especially considered in the case of insects. Appleton and Company, New York. https://www.biodiversitylibrary.org/bibliography/11353
Powell JA, Mitter C, Farrell B (1998) 20. Evolution of larval food preferences in Lepidoptera. Evol Syst Biogeogr 1:403–422
Pratt PD, Herdocia K, Valentin V, Makinson J, Purcell MF, Mattison E, Rayamajhi MB, Moran P, Raghu S (2016) Development rate, consumption, and host fidelity of Neostauropus alternus (Walker, 1855) (Lepidoptera: Notodontidae). Pan-Pacific Entomol 92(4):200–209
Pringle EL, Henning GA, Ball JB (1994) Pennington’s butterflies of southern Africa, 2nd edn. Struik Winchester, Cape Town
Pringle EG, Dirzo R, Gordon DM (2011) Indirect benefits of symbiotic coccoids for an ant-defended myrmecophytic tree. Ecology 92(1):37–46
Pringle EG, Akçay E, Raab TK, Dirzo R, Gordon DM (2013) Water stress strengthens mutualism among ants, trees, and scale insects. PLoS Biol 11(11):e1001705
Ramos RR, Freitas AV, Francini RB (2018) Defensive strategies of a noctuid Caterpillar in a Myrmecophytic plant: are Dyops larvae immune to Azteca ants? Sociobiology 65(3):397–402
Regier JC, Mitter C, Davis DR, Harrison TL, Sohn JC, Cummings MP, Zwick A, Mitter KT (2015) A molecular phylogeny and revised classification for the oldest ditrysian moth lineages (Lepidoptera: Tineoidea), with implications for ancestral feeding habits of the mega-diverse Ditrysia. Syst Entomol 40(2):409–432
Rettenmeyer CW, Rettenmeyer ME, Joseph J, Berghoff SM (2011) The largest animal association centered on one species: the army ant Eciton burchellii and its more than 300 associates. Insect Soc 58(3):281–292
Ribas CR, Schoereder JH (2004) Determining factors of arboreal ant mosaics in cerrado vegetation (Hymenoptera: Formicidae). Sociobiology 44(1):49–68
Riva F, Barbero F, Bonelli S, Balletto E, Casacci LP (2017) The acoustic repertoire of lycaenid butterfly larvae. Bioacoustics 26(1):77–90
Robbins RK (1991) Cost and evolution of a facultative mutualism between ants and lycaenid larvae (Lepidoptera). Oikos 62(3):363–369
Robbins RK, Aiello A (1982) Foodplant and oviposition records for Panamanian Lycaenidae and Riodinidae. J Lepidop Soc 36(2):65–75
Robbins RK, Lamas G, Mielke OH, Harvey DJ, Casagrande MM (1996) Taxonomic composition and ecological structure of the species-rich butterfly community at Pakitza, Parque Nacional del Manu, Peru. In: Manu: the biodiversity of southeastern Peru. The biodiversity of southeastern Peru. Smithsonian Institution Press, Washington, DC, pp 217–252
Robinson GF, Nielsen ES (1993) Tineid genera of Australia (Lepidoptera). CSIRO Publishing, Melbourne
Rocha FH, Lachaud JP, Pérez-Lachaud G (2020) Myrmecophilous organisms associated with colonies of the ponerine ant Neoponera villosa (Hymenoptera: Formicidae) nesting in Aechmea bracteata bromeliads: a biodiversity hotspot. Myrmecol News 30:73–92
Rodrigues D, Kaminski LA, Freitas AV, Oliveira PS (2010) Trade-offs underlying polyphagy in a facultative ant-tended florivorous butterfly: the role of host plant quality and enemy-free space. Oecologia 163(3):719–728
Rosi-Denadai CA, Scallion ML, Merrett CG, Yack JE (2018) Vocalization in caterpillars: a novel sound-producing mechanism for insects. J Exp Biol 221(4):jeb169466
Ross GN (1964) Life history studies on Mexican butterflies. III. Early stages of Anatole rossi, a new myrmecophilous metalmark. J Res Lepidop 3(2):81–94
Ross GN (1966) Life-history studies on Mexican butterflies. IV. The ecology and ethology of Anatole rossi, a myrmecophilous metalmark (Lepidoptera: Riodinidae). Ann Entomol Soc Am 59(5):985–1004
Rostás M (1657) Blassmann K (2009) insects had it first: surfactants as a defence against predators. Proc R Soc B Biol Sci 276:633–638
Roux O, Céréghino R, Solano PJ, Dejean A (2011) Caterpillars and fungal pathogens: two co-occurring parasites of an ant-plant mutualism. PLoS One 6(5):e20538
Saarinen EV (2005) Life history and myrmecophily of Neomyrina nivea periculosa (Lycaenidae: Theclinae). J Lepidop Soc 59(2):112–115
Saarinen EV (2006) Differences in worker caste behaviour of Oecophylla smaragdina (Hymenoptera: Formicidae) in response to larvae of Anthene emolus (Lepidoptera: Lycaenidae). Biol J Linn Soc 88(3):391–395
Saarinen EV, Daniels JC (2006) Miami blue butterfly larvae (Lepidoptera: Lycaenidae) and ants (Hymeoptera: Formicidae): new information on the symbionts of an endangered taxon. Fl Entomol 899(1):69–74
Sachs JL (2006) Cooperation within and among species. J Evol Biol 19(5):1415–1418
Sachs JL, Simms EL (2006) Pathways to mutualism breakdown. Trends Ecol Evol 21(10):585–592
Safian SZ (2012) Butterflies across the river. Report on the rapid butterfly surveys for the ‘Across The River Project’ in Sierra Leone and Liberia in 2011. Report for birdlife international and the society for the conservation of nature, Liberia. https://doi.org/10.13140/RG.2.1.1956.3607
Sáfián S (2015a) Observations on the little-known Geritola frankdaveyi Libert, 1999 (Lepidoptera: Lycaenidae: Poritiinae). Metamorphosis 26:19–23
Sáfián S (2015b) Two new Epitolini from Liberia in the genera Stempfferia Jackson, 1962 and Cephetola Libert, 1999 (Lepidoptera: Lycaenidae). Metamorphosis 26:12–19
Sáfián S, Collins SC (2014) A new Iridana Aurivillius, 1920 and a new Teratoneura Dudgeon, 1909 (Lepidoptera: Lycaenidae) from tropical Africa. Metamorphosis 25:90–96
Safian S, Collins SC (2015) Establishment of a new genus for Eresiomera paradoxa (Schultze, 1917) and related taxa (Lepidoptera: Lycaenidae) with description of two new species. Zootaxa 4018(1):124–136
Sáfián S, Larsen TB (2009) On the ecology and behavior of Cerautola crowleyi (Sharpe, 1890), Cerautola ceraunia (Hewitson, 1873) and Cerautola miranda (Staudinger, 1889) with descriptions of early stages (Lepidoptera: Lycaenidae, Epitolini). Trop Lepidop Res 1:22–28
Sala M, Casacci LP, Balletto E, Bonelli S, Barbero F (2014) Variation in butterfly larval acoustics as a strategy to infiltrate and exploit host ant colony resources. PLoS One 9(4):e94341
Salazar A, Fürstenau B, Quero C, Pérez-Hidalgo N, Carazo P, Font E, Martínez-Torres D (2015) Aggressive mimicry coexists with mutualism in an aphid. Proc Natl Acad Sci 112(4):1101–1106
Sanchez-Pena SR, Davis DR, Mueller UG (2003) A gregarious, mycophagous, myrmecophilous moth, Amydria anceps Walsingham (Lepidoptera: Acrolophidae), living in Atta mexicana (F. Smith) (Hymenoptera: Formicidae) spent fungal culture accumulations. Proc Entomol Soc Wash 105(1):186–194
Sands DP (1986) A revision of the genus Hypochrysops C. and R. Felder (Lepidoptera: Lycaenidae). Brill, Leiden
Sands DPA, Sands MC (2015) Review of variation in Acrodipsas cuprea (Sands, 1965) and A. aurata Sands, 1997 (Lepidoptera: Lycaenidae), with descriptions of a new subspecies of A. cuprea and a new species of Acrodipsas Sands from inland southern Queensland. Aust Entomol 42:197–218
Sanetra M, Fiedler K (1996) Behaviour and morphology of an aphytophagous lycaenid caterpillar: Cigaritis (Apharitis) acamas Klug, 1834 (Lepidoptera: Lycaenidae). Nota Lepidop 18(1):57–76
Santos FL, Dias FM, Leite LA, Dolibaina DR, Casagrande MM, Mielke OH (2014) Taxonomic notes on some species of Euselasia Hübner, [1819] from the “Uriiformes” group, with the description of the immature stages of Euselasia satyroides Lathy, 1926, stat. rev. (Lepidoptera: Riodinidae: Euselasiinae). Zootaxa 3869(5):501–522
Sariot M, Ginés M (2011) Biología y Ecología de los Licénidos Espanõles. Granada
Schär S, Eastwood R, Arnaldi KG, Talavera G, Kaliszewska ZA, Boyle JH, Espeland M, Nash DR, Vila R, Pierce NE (2018) Ecological specialization is associated with genetic structure in the ant-associated butterfly family Lycaenidae. Proc R Soc B Biol Sci 285:20181158
Schmid S, Schmid VS, Kamke RA, Steiner JO, Zillikens AN (2010) Association of three species of Strymon Hübner (Lycaenidae: Theclinae: Eumaeini) with bromeliads in southern Brazil. J Res Lepidop 42:50–55
Schmidt DJ, Rice SJ (2002) Association of ants with juvenile Ogyris amaryllis Hewitson (Lepidoptera: Lycaenidae) in South-Eastern Queensland. Aust J Entomol 41(2):164–169
Schmidt DJ, Grund R, Williams MR, Hughes JM (2014) Australian parasitic Ogyris butterflies: east–west divergence of highly-specialized relicts. Biol J Linn Soc 111(2):473–484
Schönrogge K, Wardlaw JC, Peters AJ, Everett S, Thomas JA, Elmes GW (2004) Changes in chemical signature and host specificity from larval retrieval to full social integration in the myrmecophilous butterfly Maculinea rebeli. J Chem Ecol 30(1):91–107
Schönrogge K, Barbero F, Casacci LP, Settele J, Thomas JA (2017) Acoustic communication within ant societies and its mimicry by mutualistic and socially parasitic myrmecophiles. Anim Behav 134:249–256
Schurian KG, Eckweiler W (2002) Beitrag zur Biologie und Ökologie von Polyommatus (Aricia) teberdina nahizericus (Eckweiler, 1978) aus der Nordosttürkei (Lepidoptera: Lycaenidae). Nachr Entomol Ver Apollo 22(4):211–214. [publication in German]
Schurian KG, Fiedler K (1994) Zur Biologie von Polyommatus (Lysandra) dezinus (De Freina & Witt) (Lepidoptera: Lycaenidae). Nachr Entomol Ver Apollo 14(4):339–353. [publication in German]
Schurian KG, Reif A (1992) Beitrag zur Biologie von Polyommatus (Aricia) isaurica (Staudinger) (Lepidoptera: Lycaenidae). Nachr Entomol Ver Apollo 12(4):255–261. [publication in German]
Schurian KG, ten Hagen W, Eckweiler W (2005) Beitrag zur Biologie und Ökologie von Polyommatus (Agrodiaetus) peilei Bethune-Baker, 1921 (Lepidoptera, Lycaenidae). Nachr Entomol Ver Apollo 26(4):197–206. [publication in German]
Seki Y, Takanami Y, Otsuka K (1991) Butterflies of Borneo (vol 2, no. 1). Tobishima, Tokyo
Sendoya SF, Oliveira PS (2015) Ant–caterpillar antagonism at the community level: interhabitat variation of tritrophic interactions in a neotropical savanna. J Anim Ecol 84(2):442–452
Sendoya SF, Oliveira PS (2017) Behavioural ecology of defence in a risky environment: caterpillars versus ants in a Neotropical savanna. Ecol Entomol 42(5):553–564
Sendoya SF, Freitas AV, Oliveira PS (2009) Egg-laying butterflies distinguish predaceous ants by sight. Am Nat 174(1):134–140
Seraphim N, Kaminski LA, Devries PJ, Penz C, Callaghan C, Wahlberg N, Silva-Brandao KL, Freitas AV (2018) Molecular phylogeny and higher systematics of the metalmark butterflies (Lepidoptera: Riodinidae). Syst Entomol 43(2):407–425
Seufert P, Fiedler K (1996) The influence of ants on patterns of colonization and establishment within a set of coexisting lycaenid butterflies in a south-east Asian tropical rain forest. Oecologia 106(1):127–136
Shapiro A (2007) Field guide to butterflies of the San Francisco Bay and Sacramento Valley regions. University of California Press, Berkeley
Shibao H, Morimoto M, Okumura Y, Shimada M (2009) Fitness costs and benefits of ant attendance and soldier production for the social aphid Pseudoregma bambucicola (Homoptera: Aphididae: Hormaphidinae). Sociobiology 54(3):673
Shimizu-kaya U, Okubo T, Yago M, Inui Y, Itioka T (2013) Myrmecoxeny in Arhopala zylda (Lepidoptera, Lycaenidae) larvae feeding on Macaranga myrmecophytes. Entomol News 123(1):63–70
Shimizu-Kaya U, Kishimoto-Yamada K, Itioka T (2015) Biological notes on herbivorous insects feeding on myrmecophytic Macaranga trees in the Lambir Hills National Park, Borneo. Contrib Biol Lab Kyoto Univ 30(2):85–125
Sielezniew M, Wlostowski M, Dziekanska I (2010) Myrmica schencki (Hymenoptera: Formicidae) as the primary host of Phengaris (Maculinea) arion (Lepidoptera: Lycaenidae) at heathlands in eastern Poland. Sociobiology 55(1):95–106
Silva NA, Duarte M, Araújo EB, Morais HC (2014) Larval biology of anthophagous Eumaeini (Lepidoptera: Lycaenidae, Theclinae) in the Cerrado of Central Brazil. J Insect Sci 14(1):184
Silveira HC, Oliveira PS, Trigo JR (2010) Attracting predators without falling prey: chemical camouflage protects honeydew-producing treehoppers from ant predation. Am Nat 175(2):261–268
Singh AP (2003) New records on the distribution and ecology of common gem butterfly, Poritia hewitsoni hewitsoni Moore from the lower Western Himalayas: a lesser known taxa. J Lepidop Soc 57(4):295–298
Smedley SR, Schroeder FC, Weibel DB, Meinwald J, Lafleur KA, Renwick JA, Rutowski R, Eisner T (2002) Mayolenes: labile defensive lipids from the glandular hairs of a caterpillar (Pieris rapae). Proc Natl Acad Sci 99(10):6822–6827
Solazzo G, Moritz RF, Settele J (2013) Choice behaviour of Myrmica rubra workers between ant larvae and larvae of their Phengaris (Maculinea) nausithous nest parasites. Insect Soc 60(1):57–64
Stadler B, Dixon AF (2005) Ecology and evolution of aphid-ant interactions. Annu Rev Ecol Evol Syst 36:345–372
Steidinger BS, Crowther TW, Liang J, Van Nuland ME, Werner GD, Reich PB, Nabuurs GJ, de Miguel S, Zhou M, Picard N, Hérault B, Zhao X, Zhang C, Routh D, GFBI consortium, Peay KG (2019) Climatic controls of decomposition drive the global biogeography of forest-tree symbioses. Nature 569(7756):404–408
Stoeffler M, Maier TS, Tolasch T, Steidle JL (2007) Foreign-language skills in rove-beetles? Evidence for chemical mimicry of ant alarm pheromones in myrmecophilous Pella beetles (Coleoptera: Staphylinidae). J Chem Ecol 33(7):1382–1392
Stradomsky BV, Fomina EA (2009) The developmental stages of some blue butterflies (Lepidoptera: Lycaenidae) of Russian south. Caucas Entomol Bull 5(2):269–272. [publication in Russian]
Styrsky JD, Eubanks MD (2007) Ecological consequences of interactions between ants and honeydew-producing insects. Proc R Soc B Biol Sci 274(1607):151–164
Susilo I, Susilo FX (2015) Preliminary study on Eublemma sp. (Eublemminae): a lepidopteran predator of Coccus viridis (Hemiptera: Coccidae) on coffee plants in Bandarlampung, Indonesia. Jurnal Hama dan Penyakit Tumbuhan Tropika 15(1):10–16
Talavera G, Kaminski LA, Freitas AV, Vila R (2016) One-note samba: the biogeographical history of the relict Brazilian butterfly Elkalyce cogina. J Biogeogr 43(4):727–737
Talavera G, Lukhtanov V, Pierce NE, Vila R (2021) DNA barcodes combined with multi-locus data of representative taxa can generate reliable higher-level phylogenies. Syst Biol. [in press]
Tartally A, Thomas JA, Anton C, Balletto E, Barbero F, Bonelli S, Bräu M, Casacci LP, Csősz S, Czekes Z, Dolek M et al (2019) Patterns of host use by brood parasitic Maculinea butterflies across Europe. Philos Trans R Soc B 374(1769):20180202
Tauber CA, Winterton SL (2014) Third instar of the myrmecophilous Italochrysa insignis (Walker) from Australia (Neuroptera: Chrysopidae: Belonopterygini). Zootaxa 3811(1):95–106
Tauber CA, Kilpatrick SK, Oswald JD (2020) Larvae of Abachrysa eureka (Banks) (Neuroptera: Chrysopidae: Belonopterygini): descriptions and a discussion of the evolution of myrmecophily in Chrysopidae. Zootaxa 4789(2):481–507
Tautz J, Fiedler K (1992) Mechanoreceptive properties of caterpillar hairs involved in mediation of butterfly-ant symbioses. Naturwissenschaften 79(12):561–563
Tennent J (1996) The butterflies of Morocco, Algeria and Tunisia. Gem Publishing Company, Wallingford
Thomas JA, Elmes GW (1993) Specialized searching and the hostile use of allomones by a parasitoid whose host, the butterfly Maculinea rebeli, inhabits ant nests. Anim Behav 45(3):593–602
Thomas JA, Elmes GW (1998) Higher productivity at the cost of increased host-specificity when Maculinea butterfly larvae exploit ant colonies through trophallaxis rather than by predation. Ecol Entomol 23(4):457–464
Thomas JA, Elmes GW (2001) Food–plant niche selection rather than the presence of ant nests explains oviposition patterns in the myrmecophilous butterfly genus Maculinea. Proc R Soc Lond Ser B Biol Sci 268(1466):471–477
Thomas JA, Elmes GW, Wardlaw JC (1998) Polymorphic growth in larvae of the butterfly Maculinea rebeli, a social parasite of Myrmica ant colonies. Proc R Soc Lond Ser B Biol Sci 265(1408):1895–1901
Thomas JA, Knapp JJ, Akino T, Gerty S, Wakamura S, Simcox DJ, Wardlaw JC, Elmes GW (2002) Parasitoid secretions provoke ant warfare. Nature 417(6888):505–506
Thomas JA, Simcox DJ, Clarke RT (2009) Successful conservation of a threatened Maculinea butterfly. Science 325(5936):80–83
Thomas JA, Elmes GW, Sielezniew M, Stankiewicz-Fiedurek A, Simcox DJ, Settele J, Schönrogge K (2013) Mimetic host shifts in an endangered social parasite of ants. Proc R Soc B Biol Sci 280(1751):20122336
Thomas CC, Tillberg CV, Schultz CB (2020) Facultative mutualism increases survival of an endangered ant-tended butterfly. J Insect Conserv 24(2):385–395
Thompson JN (2005) The geographic mosaic of coevolution. University of Chicago Press, Chicago
Torres PJ, Pomerantz AF (2016) Butterfly kleptoparasitism and first account of immature stages, myrmecophily, and bamboo host plant of the metalmark Adelotypa annulifera (Riodinidae). J Lepidop Soc 70(2):130–138
Trager MD, Daniels JC (2009) Ant tending of Miami blue butterfly larvae (Lepidoptera: Lycaenidae): partner diversity and effects on larval performance. Fl Entomol 92(3):474–482
Trager MD, Thom MD, Daniels JC (2013) Ant-related oviposition and larval performance in a myrmecophilous lycaenid. Int J Ecol 2013:152139
Travassos MA, Pierce NE (2000) Acoustics, context and function of vibrational signalling in a lycaenid butterfly–ant mutualism. Anim Behav 60(1):13–26
Travassos MA, DeVries PJ, Pierce NE (2008) A novel organ and mechanism for larval sound production in butterfly caterpillars: Eurybia elvina (Lepidoptera: Riodinidae). Trop Lepidop Res 18(1):20–23
Tshikolovets VV (2011) Butterflies of Europe and the Mediterranean area. Tshikolovets Publications, Pardubice
Ueda S, Komatsu T, Itino T, Arai R, Sakamoto H (2016) Host-ant specificity of endangered large blue butterflies (Phengaris spp., Lepidoptera: Lycaenidae) in Japan. Sci Rep 6(1):1–5
Uemura M, Perkins LE, Zalucki MP, Cribb BW (2017) Predator-prey interaction between greenhead ants and processionary caterpillars is mediated by chemical defence. Anim Behav 129:213–222
Valencia-Montoya WA, Quental TB, Tonini JFR, Talavera G, Crall JD, Lamas G, Busby RC, Carvalho APS, Morais AB, Mega NO, Romanowski HP, Liénard MA, Salzman S, Whitaker MRL, Kawahara AY, Lohman DJ, Robbins RK, Pierce NE (In review) Evolutionary tradeoffs between male secondary sexual traits revealed by a phylogeny of the hyperdiverse tribe Eumaeini (Lepidoptera: Lycaenidae). Proc R Soc B
van der Poorten GM, van der Poorten NE (2016) The butterfly fauna of Sri Lanka. Lepodon Books
van Dyck H, Regniers S (2010) Egg spreading in the ant-parasitic butterfly, Maculinea alcon: from individual behaviour to egg distribution pattern. Anim Behav 80(4):621–627
van Gasse P (2013) Butterflies of India – annotated checklist. Butterflies of India, Kruibeke
Van Mele P, Vayssieres JF, Adandonon A, Sinzogan A (2009) Ant cues affect the oviposition behaviour of fruit flies (Diptera: Tephritidae) in Africa. Physiol Entomol 34(3):256–261
Vantaux A, Roux O, Magro A, Ghomsi NT, Gordon RD, Dejean A, Orivel J (2010) Host-specific myrmecophily and myrmecophagy in the tropical coccinellid Diomus thoracicus in French Guiana. Biotropica 42(5):622–629
Vantaux A, van den Ende W, Billen J, Wenseleers T (2011) Large interclone differences in melezitose secretion in the facultatively ant-tended black bean aphid Aphis fabae. J Insect Physiol 57(12):1614–1621
Vantaux A, Roux O, Magro A, Orivel J (2012) Evolutionary perspectives on myrmecophily in ladybirds. Psyche 2012:591570
Vargas HA, Duarte M (2016) First host plant record for Strymon davara (Hewitson) (Lepidoptera, Lycaenidae) in the highly human-modified coastal valleys of the Atacama desert. Rev Bras de Entomol 60(4):352–355
Vasconcelos HL (1991) Mutualism between Maieta guianensis Aubl., a myrmecophytic melastome, and one of its ant inhabitants: ant protection against insect herbivores. Oecologia 87(2):295–298
Vegliante F, Hasenfuss I (2012) Morphology and diversity of exocrine glands in lepidopteran larvae. Annu Rev Entomol 57:187–204
Vélez-Arango AM, Vélez PD, Wolff M (2010) The life cycle of Mesosemia mevania (Hewitson 1857) (Riodinidae) in a lower montane humid forest in Colombia. J Lepidop Soc 64(1):23–28
Vila R, Bell CD, Macniven R, Goldman-Huertas B, Ree RH, Marshall CR, Balint Z, Johnson K, Benyamini D, Pierce NE (2011) Phylogeny and palaeoecology of Polyommatus blue butterflies show Beringia was a climate-regulated gateway to the New World. Proc R Soc B Biol Sci 278(1719):2737–2744
von Beeren C, Brückner A, Maruyama M, Burke G, Wieschollek J, Kronauer DJ (2018) Chemical and behavioral integration of army ant-associated rove beetles–a comparison between specialists and generalists. Front Zool 15(1):1–6
Wada A, Isobe Y, Yamaguchi S, Yamaoka R, Ozaki M (2001) Taste-enhancing effects of glycine on the sweetness of glucose: a gustatory aspect of symbiosis between the ant, Camponotus japonicus, and the larvae of the lycaenid butterfly, Niphanda fusca. Chem Senses 26(8):983–992
Wagner D (1993) Species-specific effects of tending ants on the development of lycaenid butterfly larvae. Oecologia 96(2):276–281
Wagner D (1995) Pupation site choice of a North American lycaenid butterfly: the benefits of entering ant nests. Ecol Entomol 20:384–392
Wagner D, Kurina L (1997) The influence of ants and water availability on oviposition behaviour and survivorship of a facultatively ant-tended herbivore. Ecol Entomol 22(3):352–360
Warren RJ, Elliott KJ, Giladi I, King JR, Bradford MA (2019) Field experiments show contradictory short-and long-term myrmecochorous plant impacts on seed-dispersing ants. Ecol Entomol 44(1):30–39
Weber MG, Keeler KH (2013) The phylogenetic distribution of extrafloral nectaries in plants. Ann Bot 111(6):1251–1261
Webster RP, Nielsen MC (1984) Myrmecophily in the Edward's hairstreak butterfly Satyrium edwardsii (Lycaenidae). J Lepidop Soc 38(2):124–133
Weeks JA (2003) Parasitism and ant protection alter the survival of the lycaenid Hemiargus isola. Ecol Entomol 28(2):228–232
Werner A, Kinne RK (2001) Evolution of the Na-pi cotransport systems. Am J Physiol Regul Int Comparat Physiol 280:R301–R312
Westoby M, Rice B, Shelley JM, Haig D, Kohen JL (1982) Plants' use of ant dispersal at west head, New South Wales. Ant-plant interactions. Junk Publishers, The Hague, pp 7–9
Whitaker MR, Baker CC, Salzman SM, Martins DJ, Pierce NE (2019) Combining stable isotope analysis with DNA metabarcoding improves inferences of trophic ecology. PLoS One 14(7):e0219070
Wiens JA, Chr N, Van Horne B, Ims RA (1993) Ecological mechanisms and landscape ecology. Oikos:369–380
Williams MC (1995-2020) Afrotropical butterflies. Lepid Soc Africa. https://www.lepsocafrica.org/?p=publications&s=atb
Williams MC (2006) What do the larvae of Alaena amazoula (Boisduval, 1847) (Lepidoptera: Lycaenidae: Poritiinae) feed on? Metamorphosis 17(4):140–150
Williams MC, Joannou JG (1996) Observations on the oviposition behaviour and caterpillars of Thestor basutus capeneri Dickson (Lepidoptera: Lycaenidae) in South Africa. Metamorphosis 7:12–16
Witek M, Sliwinska EB, Skorka P, Nowicki P, Settele J, Woyciechowski M (2006) Polymorphic growth in larvae of Maculinea butterflies, as an example of biennialism in myrmecophilous insects. Oecologia 148(4):729–733
Witek M, Śliwińska E, Skorka P, Nowicki P, Wantuch M, Vrabec V, Settele J, Woyciechowski M (2008) Host ant specificity of large blue butterflies Phengaris (Maculinea) (Lepidoptera: Lycaenidae) inhabiting humid grasslands in east-Central Europe. Eur J Entomol 105(5):871–877
Witek M, Skórka P, Śliwińska EB, Nowicki P, Moroń D, Settele J, Woyciechowski M (2011) Development of parasitic Maculinea teleius (Lepidoptera, Lycaenidae) larvae in laboratory nests of four Myrmica ant host species. Insect Soc 58(3):403–411
Wynhoff I, Grutters M, Van Langevelde F (2008) Looking for the ants: selection of oviposition sites by two myrmecophilous butterfly species. Anim Biol 58(4):371–388
Wynhoff I, Bakker RB, Oteman B, Arnaldo PS, van Langevelde F (2015) Phengaris (Maculinea) alcon butterflies deposit their eggs on tall plants with many large buds in the vicinity of Myrmica ants. Insect Conserv Divers 8(2):177–188
Yack JE, Smith ML, Weatherhead PJ (2001) Caterpillar talk: acoustically mediated territoriality in larval Lepidoptera. Proc Natl Acad Sci 98(20):11371–11375
Yadav C, Guedes RN, Matheson SM, Timbers TA, Yack JE (2017) Invitation by vibration: recruitment to feeding shelters in social caterpillars. Behav Ecol Sociobiol 71(3):51. https://doi.org/10.1007/s00265-017-2280-x
Yago M, Miyagawa T, Yokoyama J, Williams M (2010) Life history of the Guatemalan copper, Iophanus pyrrhias (Godman & Salvin) (Lepidoptera, Lycaenidae). Trans Lepidop Soc Jpn 60(4):259–267. https://doi.org/10.18984/lepid.60.4_259
Yamagushi S, Shirozu T (1988) The life histories of five Myrmecophilous Lycaenid butterflies of Japan. Kodansha, Tokyo
Yao I (2014) Costs and constraints in aphid-ant mutualism. Ecol Res 229(3):383–391. https://doi.org/10.1007/s11284-014-1151-4
Young AM (1978) Possible evolution of mutualism between Mechanitis caterpillars and an ant in northeastern Costa Rica. Biotropica 10:77–78. https://doi.org/10.2307/2388114
Youngsteadt E, Devries PJ (2005) The effects of ants on the entomophagous butterfly caterpillar Feniseca tarquinius, and the putative role of chemical camouflage in the Feniseca–ant interaction. J Chem Ecol 31(9):2091–2109. https://doi.org/10.1007/s10886-005-6079-2
Zanuncio JC, Torres JB, Sediyama CA, Pereira FF, Pastori PL, Wermelinger ED, Ramalho FS (2009) Mortality of the defoliator Euselasia eucerus (Lepidoptera: Riodinidae) by biotic factors in an Eucalyptus urophylla plantation in Minas Gerais State, Brazil. Ann Acad Bras Ciê 81(1):61–66. https://doi.org/10.1590/S0001-37652009000100008
Zanuncio JC, Soares MA, Zanuncio TV, Mielke OH, Ramalho FD, Júnior SL, Wilcken CF (2013) Euselasia hygenius occulta (Riodininae): first report of feeding on Psidium guajava (Myrtaceae) in Minas Gerais State, Brazil. J Lepidop Soc 67(3):221–224. https://doi.org/10.18473/lepi.v67i3.a8
Zemeitat DS (2017) Evolution of cooperative behaviour in Australian lycaenid butterflies and ants. PhD Thesis, University of Melbourne
Zhou L, Zhuang H (eds) (2018) Zephyrus hairstreaks from China I. Hong Kong Lepidopterists’ Society Limited, Hong Kong
Acknowledgments
We thank Robert Marquis and Suzanne Koptur for their encouragement and patience in organizing and editing this volume. Bert Hölldobler and Roger L. Kitching provided inspiration and guidance in improving our understanding of myrmecophiles. Chris Baker, Colin E. Congdon, Alan Heath, Masaru Hojo, Daniel H. Janzen and Winnie Hallwachs, Daniel Kronauer, Dino Martins, David Nash, Piotr Naskrecki, Lucas Kaminski, and Joe Parker helped resolve some key concepts and clarify critical ambiguities in published information. We also thank Marianne Espeland, Michael Braby, and Jaret Daniels for their insightful comments and criticisms of the manuscript. David J. Lohman gave expert advice and assisted in numerous ways, especially in helping to consider how to structure the data for Table 2. João Tonini gave advice about data gathering for Table 2 and helped prepare Fig. 23. Andrew Berry gave advice and support throughout. Numerous naturalists, scientists, and photographers generously gave permission to reproduce their images (their respective names accompany each figure). Takashi Komatsu and Takao Itino kindly provided photographs from their study of the Japanese arctiid moth, Nudina artaxidia, associating with Lasius ants and homopterans, for our chapter frontispiece. Support for this research came from NSF DEB-1541560 to NEP.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2022 The Author(s)
About this chapter
Cite this chapter
Pierce, N.E., Dankowicz, E. (2022). The Natural History of Caterpillar-Ant Associations. In: Marquis, R.J., Koptur, S. (eds) Caterpillars in the Middle. Fascinating Life Sciences. Springer, Cham. https://doi.org/10.1007/978-3-030-86688-4_11
Download citation
DOI: https://doi.org/10.1007/978-3-030-86688-4_11
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-86687-7
Online ISBN: 978-3-030-86688-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)