Abstract
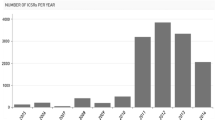
Pharmacovigilance in Africa has grown sharply this millennium with the number of African countries joining the World Health Organisation (WHO) Programme for International Drug Monitoring having increased from just 5 in the year 2000 to 35 in 2017. However, published information indicates that Africa’s contribution of individual case safety reports (ICSRs) to the WHO ICSR database (VigiBase) is paltry currently standing at less than 1% of the >14 million ICSRs in VigiBase. Moreover, there is little evidence of African countries collecting, analyzing, and using data from their settings to inform pharmacovigilance and drug safety decisions in their own countries. The huge doses of medicine and vaccines deployed for public health programs including those against malaria, tuberculosis, and HIV/AIDS as well as those for infant immunization against preventable diseases means that there is opportunity to collect real world data in relation to these medicines and vaccines. Spontaneous reporting may not necessarily be the best approach in the various African countries considering the high under-reporting associated with all spontaneous reporting schemes globally. However, there are opportunities to utilize more active pharmacovigilance approaches including cohort event monitoring and targeted spontaneous reporting to improve collection and use of safety data in Africa to improve patient care, especially in public health programs in Africa.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
World Health Assembly (1963) Clinical and pharmacological evaluation of drugs
Ampadu HH, Hoekman J, de Bruin ML, Pal SN, Olsson S, Sartori D et al (2016) Adverse drug reaction reporting in Africa and a comparison of individual case safety report characteristics between Africa and the rest of the world: analyses of spontaneous reports in VigiBase®. Drug Saf 39(4):335–345
Isah AO, Pal SN, Olsson S, Dodoo A, Bencheikh RS (2012) Specific features of medicines safety and pharmacovigilance in Africa. Ther Adv Drug Saf 3(1):25–34
Rovira J (2002) Pharmaceuticals, globalization and developing countries: recent developments and challenge. Pharm Policy Law 5:7–10
Pirmohamed M, Atuah KN, Dodoo AN, Winstanley P, Pirmohamed M, Atuah KN, Dodoo AN, Winstanley P (2007) Pharmacovigilance in developing countries. BMJ 335(7618):462
Xueref S, Daviaud J, Pal S (2013) The Global Fund and pharmacovigilance systems in resource-limited settings. Lancet 381(9875):1360
Stergachis A, Bartlein RJ, Dodoo A, Nwokike J, Kachur SP (2010) A situational analysis of pharmacovigilance plans in the Global Fund Malaria and U.S. President’s Malaria Initiative proposals. Malar J 9:148
Miller V, Nwokike J, Stergachis A (2012) Pharmacovigilance and global HIV/AIDS. Curr Opin HIV AIDS 7(4):299–304
Adjei A, Narh-Bana S, Amu A, Kukula V, Nagai RA, Owusu-Agyei S et al (2016) Treatment outcomes in a safety observational study of dihydroartemisinin/piperaquine (Eurartesim(®)) in the treatment of uncomplicated malaria at public health facilities in four African countries. Malar J 15:43
Available from: http://www.who.int/vaccine_safety/initiative/tools/CIOMS_report_WG_vaccine.pdf. Cited
Kukula VA, Dodoo AA, Akpakli J, Narh-Bana SA, Clerk C, Adjei A et al (2015) Feasibility and cost of using mobile phones for capturing drug safety information in peri-urban settlement in Ghana: a prospective cohort study of patients with uncomplicated malaria. Malar J 14:411
Kimutai R, Musa AM, Njoroge S, Omollo R, Alves F, Hailu A et al (2017) Safety and effectiveness of sodium stibogluconate and paromomycin combination for the treatment of visceral leishmaniasis in eastern Africa: results from a pharmacovigilance programme. Clin Drug Investig 37(3):259–272
Birbal S, Dheda M, Ojewole E, Oosthuizen F (2016) Adverse drug reactions associated with antiretroviral therapy in South Africa. Afr J AIDS Res 15(3):243–248
Masimirembwa C, Dandara C, Leutscher PD (2016) Rolling out efavirenz for HIV precision medicine in Africa: are we ready for pharmacovigilance and tackling neuropsychiatric adverse effects? OMICS 20(10):575–580
Cliff-Eribo KO, Sammons H, Choonara I (2016) Systematic review of paediatric studies of adverse drug reactions from pharmacovigilance databases. Expert Opin Drug Saf 15(10):1321–1328
Rachlis B, Karwa R, Chema C, Pastakia S, Olsson S, Wools-Kaloustian K et al (2016) Targeted spontaneous reporting: assessing opportunities to conduct routine pharmacovigilance for antiretroviral treatment on an international scale. Drug Saf 39(10):959–976
Diap G, Amuasi J, Boakye I, Sevcsik AM, Pecoul B (2010) Anti-malarial market and policy surveys in sub-Saharan Africa. Malar J 9(Suppl 1):S1
Kuemmerle A, Dodoo AN, Olsson S, Van Erps J, Burri C, Lalvani PS (2011) Assessment of global reporting of adverse drug reactions for anti-malarials, including artemisinin-based combination therapy, to the WHO Programme for International Drug Monitoring. Malar J 10:57
World Health Organisation (2003) WHO Training Workshop on Pharmacovigilance: basic introduction and specifics for malaria programmes, 2003, p 11
McEwen J (2012) Artesunate- and amodiaquine-associated extrapyramidal reactions: a series of 49 cases in VigiBase™. Drug Saf 35(8):667–675
Suku CK, Hill G, Sabblah G, Darko M, Muthuri G, Abwao E et al (2015) Experiences and lessons from implementing cohort event monitoring programmes for antimalarials in four African countries: results of a questionnaire-based survey. Drug Saf 38(11):1115–1126
Dodoo AN, Fogg C, Nartey ET, Ferreira GL, Adjei GO, Kudzi W et al (2014) Profile of adverse events in patients receiving treatment for malaria in urban Ghana: a cohort-event monitoring study. Drug Saf 37(6):433–448
Dodoo ANO, Fogg C, Asiimwe A, Nartey ET, Kodua A, Tenkorang O et al (2009) Pattern of drug utilization for treatment of uncomplicated malaria in uncomplicated malaria in urban Ghana following national treatment policy change to artemisinin-combination therapy. Malar J 8:2
Peters PJ, Thigpen MC, Parise ME, Newman RD (2007) Safety and toxicity of sulfadoxine/pyrimethamine: implications for malaria prevention in pregnancy using intermittent preventive treatment. Drug Saf 30(6):481–501
Aponte JJ, Schellenberg D, Egan A, Breckenridge A, Carneiro I, Critchley J et al (2009) Efficacy and safety of intermittent preventive treatment with sulfadoxine-pyrimethamine for malaria in African infants: a pooled analysis of six randomised, placebo-controlled trials. Lancet 2009:1533–1542
Snow RW (2016) Seasonal malaria chemoprevention: an evolving research paradigm. PLoS Med 13(11):e1002176
(2014) Artesunate. The standard intravenous treatment for severe attacks of malaria. Prescrire Int 23(154):260
Gosling R, von Seidlein L (2016) The future of the RTS,S/AS01 malaria vaccine: an alternative development plan. PLoS Med 13(4):e1001994
Twomey PS, Smith BL, McDermott C, Novitt-Moreno A, McCarthy W, Kachur SP et al (2015) Intravenous artesunate for the treatment of severe and complicated malaria in the United States: clinical use under an investigational new drug protocol. Ann Intern Med 163(7):498–506
Kharsany AB, Karim QA (2016) HIV infection and AIDS in sub-Saharan Africa: current status, challenges and opportunities. Open AIDS J 10:34–48
Tetteh RA, Nartey ET, Lartey M, Mantel-Teeuwisse AK, Leufkens HG, Nortey PA et al (2015) Adverse events and adherence to HIV post-exposure prophylaxis: a cohort study at the Korle-Bu Teaching Hospital in Accra, Ghana. BMC Public Health 15:573
Tetteh RA, Nartey ET, Lartey M, Mantel-Teeuwisse AK, Leufkens HG, Yankey BA et al (2016) Association between the occurrence of adverse drug events and modification of first-line highly active antiretroviral therapy in Ghanaian HIV patients. Drug Saf 39(11):1139–1149
Pal SN, Duncombe C, Falzon D, Olsson S (2013) WHO strategy for collecting safety data in public health programmes: complementing spontaneous reporting systems. Drug Saf 36(2):75–81
Violari A, Cotton MF, Gibb DM, Babiker AG, Steyn J, Madhi SA et al (2008) Early antiretroviral therapy and mortality among HIV-infected infants. N Engl J Med 359(21):2233–2244
Cotton MF, Violari A, Otwombe K, Panchia R, Dobbels E, Rabie H et al (2013) Early time-limited antiretroviral therapy versus deferred therapy in South African infants infected with HIV: results from the children with HIV early antiretroviral (CHER) randomised trial. Lancet 382(9904):1555–1563
Tymchuk CN, Currier JS (2008) The safety of antiretroviral drugs. Expert Opin Drug Saf 7(1):1–4
Falzon D, Hill G, Pal SN, Suwankesawong W, Jaramillo E (2014) Pharmacovigilance and tuberculosis: applying the lessons of thioacetazone. Bull World Health Organ 92(12):918–919
Wong EB, Cohen KA, Bishai WR (2013) Rising to the challenge: new therapies for tuberculosis. Trends Microbiol 21(9):493–501
Skrahina A, Hurevich H, Falzon D, Zhilevich L, Rusovich V, Dara M et al (2016) Bedaquiline in the multidrug-resistant tuberculosis treatment: Belarus experience. Int J Mycobacteriol 5(Suppl 1):S62–SS3
Guglielmetti L, Le Dû D, Jachym M, Henry B, Martin D, Caumes E et al (2015) MDR-TB Management Group of the French National Reference Center for Mycobacteria and the Physicians of the French MDR-TB Cohort. Compassionate use of bedaquiline for the treatment of multidrug-resistant and extensively drug-resistant tuberculosis: interim analysis of a French cohort. Clin Infect Dis 60(2):188–194
Chocarro L, Duclos P, Senouci K, Southern J (2011) Consultation on interactions between National Regulatory Authorities and National Immunization Technical Advisory Groups. Expert Rev Vaccines 10(9):1265–1270
World Health Organisation (2012) The global vaccine safety blueprint
Letourneau M, Wells G, Walop W, Duclos P (2008) Improving global monitoring of vaccine safety: a quantitative analysis of adverse event reports in the WHO Adverse Reactions Database. Vaccine 26(9):1185–1194
Dodoo A, Renner L, van Grootheest AC, Labadie J, Antwi-Agyei KO, Hayibor S et al (2007) Safety monitoring of a new pentavalent vaccine in the expanded programme on immunisation in Ghana. Drug Saf 30(4):347–356
Chaibou MS, Bako H, Salisou L, Yaméogo TM, Sambo M, Kim SH et al (2010) Monitoring adverse events following immunization with a new conjugate vaccine against group A meningococcus in Niger. Vaccine 30(35):5229–5234
Laserson KF, Nyakundi D, Feikin DR, Nyambane G, Cook E, Oyieko J et al (2012) Safety of the pentavalent rotavirus vaccine (PRV), RotaTeq(®), in Kenya, including among HIV-infected and HIV-exposed infants. Vaccine 30(Suppl 1):A61–A70
Odusanya OO, Kuyinu YA, Kehinde OA, Shafi F, François N, Yarzabal JP et al (2014) Safety and immunogenicity of 10-valent pneumococcal nontypeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) in Nigerian children: booster dose and 2-dose catch-up regimens in the second year of life. Hum Vaccin Immunother 10(3):757–766
Mugo N, Ansah NA, Marino D, Saah A, Garner EI (2015) Evaluation of safety and immunogenicity of a quadrivalent human papillomavirus vaccine in healthy females between 9 and 26 years of age in sub-Saharan Africa. Hum Vaccin Immunother 11(6):1323–1330
Berhe DF, Juhlin K, Star K, Beyene KG, Dheda M, Haaijer-Ruskamp FM et al (2015) Adverse drug reaction reports for cardiometabolic drugs from sub-Saharan Africa: a study in VigiBase. Tropical Med Int Health 20(6):797–806
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Science+Business Media, LLC, part of Springer Nature
About this protocol
Cite this protocol
Ampadu, H.H., Esseku, Y., Dodoo, A.N.O. (2018). Evidence-Based Pharmacovigilance for Medicines Used in Public Health Programs in Africa. In: Bate, A. (eds) Evidence-Based Pharmacovigilance. Methods in Pharmacology and Toxicology. Humana Press, New York, NY. https://doi.org/10.1007/978-1-4939-8818-1_10
Download citation
DOI: https://doi.org/10.1007/978-1-4939-8818-1_10
Published:
Publisher Name: Humana Press, New York, NY
Print ISBN: 978-1-4939-8816-7
Online ISBN: 978-1-4939-8818-1
eBook Packages: Springer Protocols