Abstract
Here, we describe an in vivo model in which antisense oligonucleotides were preclinically evaluated in reconstituted patient and healthy control skin. The aim was to investigate the effect of antisense oligonucleotides upon local or systemic administration. This allows for clinically relevant evaluation of antisense oligonucleotides in an in vivo setting. In this model, primary human keratinocytes and fibroblasts were placed into silicone grafting chambers, implanted onto the back of athymic nude mice. After sufficient cells were expanded, within a few weeks, human skin grafts were generated with a high success rate. These mice bearing grafts were subsequently treated with antisense oligonucleotides targeting exon 105 of the COL7A1 gene which encodes type VII collagen. Patients completely lacking expression of type VII collagen develop severe blistering of skin and mucosa, i.e., recessive dystrophic epidermolysis bullosa. In this chapter, we describe the in vivo model used for the preclinical evaluation of antisense oligonucleotides as therapeutic approach for recessive dystrophic epidermolysis bullosa.
You have full access to this open access chapter, Download protocol PDF
Similar content being viewed by others
Keywords
1 Introduction
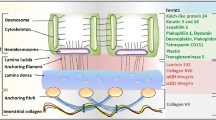
Antisense oligonucleotide (ASO)-mediated exon skipping has been shown to have great potential as therapeutic approach for the devastating heritable skin blistering disease dystrophic epidermolysis bullosa (DEB) [1, 2]. Severe recessive DEB (RDEB-gen sev) is caused by biallelic null variants in the COL7A1 gene, which encodes type VII collagen (C7). C7 is an extracellular matrix molecule that secures attachment of the epidermis to the dermis by the formation of anchoring fibrils. Imaginably, the complete absence of C7 results in severe blistering of skin and mucosa and early demise [3]. The aim of ASO-mediated exon skipping is to remove the exon from the transcriptome in which the disease-causing variant resides. As a result, a slightly shorter protein is expressed which is functional [4]. In this chapter, we describe the evaluation of ASO targeting skin in an in vivo setting. The model is based on the cell sorting principle, in which cells are of the same cell type cluster; in this case, primary human fibroblasts and keratinocytes [5]. Athymic nude mice were used for several reasons, foremost, these mice are highly suitable for xenografting as the mice do not possess a thymus and therefore do not display graft rejection. Second, morphological changes, like pigmentation or blistering, can be easily observed as the mice have little to no fur. These mice are bred and kept in individually ventilated cages (IVC), as they are vulnerable to pathogens.
Initially, a submerged culture was established in silicone grafting chambers that were implanted onto the back of these mice. This submerged culture allows for proliferation, clustering, and attachment of fibroblasts and keratinocytes onto the muscle fascia. After 10 days, the silicone grafting chamber is removed, which creates an air–liquid interface. It is well known that an air–liquid interface is essential for differentiation of the keratinocytes and form a stratum corneum. These principles are similar to the principles of 3D skin-equivalent culture in vitro.
The major advantage of this model is the ability to evaluate ASOs that are systemically administered. Additionally, this model allows treatment for longer periods than in vitro. The major disadvantage of this model is the need for, in comparison with in vitro 3D-culture , high numbers of patient cells. Primary cells were used; however, cell lines or iPSC-derived cells could be used as well. When using primary cells, the lower the passage, the better-defined strata of skin as the proliferative potential and differential is the highest. In this chapter, we describe the grafting model that was used to evaluate ASO-mediated exon skipping upon systemic treatment using primary cultured fibroblasts and keratinocytes.
2 Materials
-
1.
Primary cultured human skin fibroblasts (5–6 × 106 per animal).
-
2.
Primary cultured human skin keratinocytes (5–6 × 106 per animal).
-
3.
Phosphate-buffered saline (PBS).
-
4.
HEPES-buffered saline solution (HBSS).
-
5.
Hyclone fetal bovine serum (Chelex 100 treated) (see Note 1).
-
6.
Grafting medium (1:1 DMEM:Ham-F12 + 5% FBS).
-
7.
Silicone grafting chamber (made by medical instrument maker).
-
8.
Surgical instruments: scissors (Metzenbaum), tweezers, forceps.
-
9.
Suture wire (5-0).
-
10.
Biotechnical surgery equipment.
-
11.
Buprenorphine (0.01 mg/ml).
-
12.
Chlorhexidine 2% for topical application.
-
13.
70% alcohol.
-
14.
Weighing scale for mice.
3 Methods
3.1 Anesthesia and Handling
Due to the wide variability in laboratory animal practices and surgical instruments used in laboratories, we generally describe the anesthesia procedure. Anesthesia of the animal was induced using 5% isoflurane gas in a closed induction chamber designed for mice with continuous flow of 5% isoflurane gas carried by oxygen. After induction, the animal was transferred onto a heated pad where anesthesia was maintained using 2% isoflurane gas by placing the head of the mouse into a breathing conduit through which the gas continuously flows. All handling and surgery were performed inside a laminar biohazard flow cabinet.
3.2 Surgical Procedure
-
1.
Harvest 5–6 × 106 fibroblasts and 5–6 × 106 keratinocytes. Spin down at 200 × g and remove supernatant. Resuspend and pool pellets in 4 ml grafting medium. Spin down at 200 × g and remove supernatant. Keep pellet on ice and proceed to the animal laboratory.
-
2.
Take the IVC cage containing the animal and spray it down with 70% alcohol, then place the cage in the flow cabinet.
-
3.
Open the cage and transfer the mouse into the induction chamber containing 5% isoflurane gas and write the time in your log as the start of the operation.
-
4.
Once induced, weigh the mouse and transfer the mouse onto the heated pad and place the head of the mouse into the breathing conduit. Make sure the mouse is under by pinching a paw. Only when completely under anesthesia, continue.
-
5.
With a sterile gauze use chlorhexidine to sanitize the neck and tale base of the mouse .
-
6.
Inject 10 μl/g buprenorphine at the tail base subcutaneously.
-
7.
Using a permanent marker, draw an 8–10 mm diameter circle between the shoulder blades loose skin.
-
8.
Using a tweezer, lift the skin in the middle of the circle and excise a full skin thickness circle revealing the muscle fascia (Fig. 1a).
-
9.
Once removed, use the surgical scissors to separate the skin from the fascia surrounding the excision.
-
10.
Insert the silicone grafting chamber in the excised area.
-
11.
Once the grafting chamber is implanted, the chamber should be securely held in place by the surrounding skin. If necessary, suture the skin surrounding the chamber together, thereby securing the chamber in place.
-
12.
Resuspend the pellet in grafting medium and pipet gently dropwise into the grafting chamber. Write the time in your log (Fig. 1b).
-
13.
Reduce the isoflurane to 1.75% (see Note 2) and cover the lower part of the mouse with a few gauzes to make sure the mouse remains warm.
-
14.
40 min after the cells are pipetted onto the muscle facia in the grafting chamber, gently place the mouse back into the IVC cage, close the lid, and monitor the mouse as it recovers from anesthesia.
-
15.
Every 8 h, the mouse is treated with 10 μl/g buprenorphine for 48 h. After 48 h, the mice should be monitored daily for behavior (see Note 3) (Fig. 1c).
Grafting procedure. In this figure, the steps of the grafting procedure are explained. (a) First, a full-thickness skin excision is made. (b) Then the silicone grafting chamber is implanted, followed by injection of the cells in grafting medium in the chamber. (c) Subsequently, the chamber will be filled with wound fluid and after (d) 7 days, the grafting chamber is removed. (e) A scab will be formed on the wound which will (f) fall off around 10 days after removal of the chamber and a patch of human skin is revealed
3.3 Removal of the Grafting Chambers
Ten days post operation, the grafting chambers are removed.
-
1.
Induce anesthesia as described above. Carefully using precision tweezers release the graft from the inside of the grafting chamber (see Note 4) and the grafting chamber from the skin surrounding the chamber. Remove sutures if necessary.
-
2.
Once free, carefully remove the grafting chamber using tweezers, revealing the white-yellow graft in the middle (Fig. 1d).
-
3.
The mouse is kept under anesthesia for 10 min after removal by 2% isoflurane gas in oxygen.
-
4.
Gently place the mouse back into the IVC cage and monitor the mouse as it comes back to esthesia.
-
5.
Within 24 h, a scab will form (Fig. 1e). Generally, the scab will fall off after a week (see Note 5) revealing the human skin graft (Fig. 1f).
4 Notes
-
1.
Hyclone fetal bovine serum is chelated using 4 g Chelex 100 (Bio-Rad)/100 mL FBS. Left to rotate overnight at 4 °C, paper filtrated to remove residual resin, then sterile filtered and kept in aliquots at −20 °C. It is essential to use low calcium serum, as calcium will induce premature differentiation of keratinocytes.
-
2.
It is essential to monitor mice under isoflurane anesthesia. Try to find the minimum required to sustain anesthesia, as the mice are sensitive to isoflurane.
-
3.
Injections are given under short general anesthesia to minimize the risk of damaging the chambers/grafts. The mice are housed individually, as mice will eat the silicone grafting chamber. Additionally, remove cage enrichment where the chamber can get stuck behind, e.g., small houses or tubes. Sterilized tissues can be used as replacement for cage enrichment. The mice will build burrows with them, and they cannot damage the grafting chamber or graft.
-
4.
Make sure the graft and skin are completely cleared from the chamber. Otherwise, lifting the chamber could remove (part of) the graft.
-
5.
Do not physically remove the scab. The time required for the scab to fall off varies. Usually between 7 and 10 days.
References
Bremer J, Bornert O, Nystrom A, Gostynski A, Jonkman MF, Aartsma-Rus A, van den Akker PC, Pasmooij AM (2016) Antisense oligonucleotide-mediated exon skipping as a systemic therapeutic approach for recessive dystrophic epidermolysis bullosa. Mol Ther Nucleic Acids 5(10):e379. https://doi.org/10.1038/mtna.2016.87
Bremer J, van der Heijden EH, Eichhorn DS, Meijer R, Lemmink HH, Scheffer H, Sinke RJ, Jonkman MF, Pasmooij AMG, Van den Akker PC (2019) Natural exon skipping sets the stage for exon skipping as therapy for dystrophic epidermolysis bullosa. Mol Ther Nucleic Acids 18:465–475. https://doi.org/10.1016/j.omtn.2019.09.009
Has C, Bauer JW, Bodemer C, Bolling MC, Bruckner-Tuderman L, Diem A, Fine JD, Heagerty A, Hovnanian A, Marinkovich MP, Martinez AE, McGrath JA, Moss C, Murrell DF, Palisson F, Schwieger-Briel A, Sprecher E, Tamai K, Uitto J, Woodley DT, Zambruno G, Mellerio JE (2020) Consensus reclassification of inherited epidermolysis bullosa and other disorders with skin fragility. Br J Dermatol 183(4):614–627. https://doi.org/10.1111/bjd.18921
Bornert O, Kuhl T, Bremer J, van den Akker PC, Pasmooij AM, Nystrom A (2016) Analysis of the functional consequences of targeted exon deletion in COL7A1 reveals prospects for dystrophic epidermolysis bullosa therapy. Mol Ther 24(7):1302–1311. https://doi.org/10.1038/mt.2016.92
Wang CK, Nelson CF, Brinkman AM, Miller AC, Hoeffler WK (2000) Spontaneous cell sorting of fibroblasts and keratinocytes creates an organotypic human skin equivalent. J Invest Dermatol 114(4):674–680. https://doi.org/10.1046/j.1523-1747.2000.00938.x
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2022 The Author(s)
About this protocol
Cite this protocol
Bremer, J., van den Akker, P.C. (2022). In Vivo Models for the Evaluation of Antisense Oligonucleotides in Skin. In: Arechavala-Gomeza, V., Garanto, A. (eds) Antisense RNA Design, Delivery, and Analysis. Methods in Molecular Biology, vol 2434. Humana, New York, NY. https://doi.org/10.1007/978-1-0716-2010-6_21
Download citation
DOI: https://doi.org/10.1007/978-1-0716-2010-6_21
Published:
Publisher Name: Humana, New York, NY
Print ISBN: 978-1-0716-2009-0
Online ISBN: 978-1-0716-2010-6
eBook Packages: Springer Protocols