Abstract
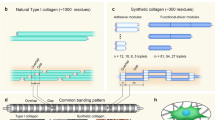
Collagen represents the major structural protein of the extracellular matrix. The desired mechanical and biological performances of collagen that have led to its broad applications as a building block in a great deal of fields, such as tissue engineering, drug delivery, and nanodevices. The most direct way to obtain collagen is to separate and extract it from biological tissues, but these top-down methods are usually cumbersome, and the structure of collagen is usually destroyed during the preparation process. Moreover, there is currently no effective method to separate some scarce collagens (such as collagen from human beings). Alternatively, bottom-up assembly methods have been developed to obtain collagen assembly or their analogs. The collagen used in this type of method is usually obtained by genetic recombination. A distinct advantage of gene recombination is that the sequence structure of collagen can be directly customized, so its assembly mode can be regulated at the primary structure level, and then a collagen assembly with a predesigned configuration can be achieved. Additionally, insights into the assembly behavior of these specific structures provide a rational approach to understand the pathogenic mechanisms of collagen-associated diseases, such as diabetes. In this chapter, Type I collagen is used as an example to introduce the key methods and procedures of collagen recombination, and on this basis, we will introduce in detail the experimental protocols for further assembly of these recombinant proteins to specific structures, such as fibril.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Di Lullo GA, Sweeney SM, Korkko J, Ala-kokko L, San Antonio JD (2002) Mapping the ligand-binding sites and disease-associated mutations on the most abundant protein in the human, type I collagen. J Biol Chem 277(6):4223–4231
Sionkowska A, Skrzynski S, Smiechowski K, Kolodziejczak A (2017) The review of versatile application of collagen. Polym Adv Technol 28(1):4–9
Cen L, Liu W, Cui L, Zhang W, Cao Y (2008) Collagen tissue engineering: development of novel biomaterials and applications. Pediatr Res 63:492–496
Song WK, Liu D, Sun L, Li B, Hou H (2019) Physicochemical and biocompatibility properties of type I collagen from the skin of Nile tilapia (Oreochromis niloticus) for biomedical applications. Mar Drugs 17(3):137–151
Ottani V, Martini D, Franchi M, Ruggeri A, Raspanti M (2002) Hierarchical structures in fibrillar collagens. Micron 33(7-8):587–596
Liu Y, Kim Y, Dai L, Li N, Khan SO, Pashley DH, Tay FR (2011) Hierarchical and non-hierarchical mineralisation of collagen. Biomaterials 32(5):1291–1300
O’Leary LE, Fallas JA, Bakota EL, Kang MK, Hartgerink JD (2011) Multi-hierarchical self-assembly of a collagen mimetic peptide from triple helix to nanofibre and hydrogel. Nat Chem 3(8):821–828
Fratzl P (2008) Collagen: structure and mechanics. Springer US, New York
Parenteau-Bareil R, Gauvin R, Berthod F (2010) Collagen-based biomaterials for tissue engineering applications. Materials 3(3):1863–1887
Hafner AE, Gyori NG, Bench CA, Davis L, Saric A (2020) Modelling fibrillogenesis of collagen-mimetic molecules. 119(9):1791-1799
Abou Neel EA, Bozec L, Knowles JC, Syed O, Mudera V, Day R, Hyun JK (2013) Collagen--emerging collagen based therapies hit the patient. Adv Drug Deliv Rev 65(4):429–456
Gomes S, Leonor IB, Mano JF, Reis RL, Kaplan DL (2012) Natural and genetically engineered proteins for tissue engineering. Prog Polym Sci 37(1):1–17
Hulmes DJ, Jesior JC, Miller A, Berthet-Colominas C, Wolff C (1981) Electron microscopy shows periodic structure in collagen fibril cross sections. Proc Natl Acad Sci U S A 78(6):3567–3571
Williams BR, Gelman RA, Poppke DC, Piez KA (1978) Collagen fibril formation. Optimal in vitro conditions and preliminary kinetic results. J Biol Chem 253(18):6578–6585
Ling S, Chen W, Fan Y, Zheng K, Jin K, Yu H, Buehler MJ, Kaplan DL (2018) Biopolymer nanofibrils: structure, modeling, preparation, and applications. Prog Polym Sci 85:1–56
Li Y, Asadi A, Monroe MR, Douglas E (2009) pH effects on collagen fibrillogenesis in vitro: electrostatic interactions and phosphate binding. Mater Sci Eng C 29(5):1643–1649
Myllyharju J, Nokelainen M, Vuorela A, Kivirikko KI (2000) Expression of recombinant human type i-iii collagens in the yeast Pichia pastoris. Biochem Soc Trans 28(3):353–357
Parkinson J, Kadler KE, Brass A (1995) Simple physical model of collagen fibrillogenesis based on diffusion limited aggregation. J Mol Biol 247(4):823–831
Foo CWP, Kaplan DL (2002) Genetic engineering of fibrous proteins: spider dragline silk and collagen. Adv Drug Deliv Rev 54(8):1131–1143
Liu W, Merrett K, Griffith M, Fagerholm P, Dravida S, Heyne B, Scaiano JC, Watsky MA, Shinozaki N, Lagali N, Munger R, Li F (2008) Recombinant human collagen for tissue engineered corneal substitutes. Biomaterials 29(9):1147–1158
Leikin S, Rau DC, Parsegian VA (1994) Direct measurement of forces between self-assembled proteins: temperature-dependent exponential forces between collagen triple helices. Proc Natl Acad Sci U S A 91(1):276–280
Jiang F, Horber H, Howard J, Muller DJ (2004) Assembly of collagen into microribbons: effects of pH and electrolytes. J Struct Biol 148(3):268–278
Han R, Zwiefka A, Caswell CC, Xu Y, Keene DR, Lukomska E, Zhao Z, Hook M, Lukomski S (2006) Assessment of prokaryotic collagen-like sequences derived from streptococcal Scl1 and Scl2 proteins as a source of recombinant GXY polymers. Appl Microbiol Biotechnol 72(1):109–115
Nokelainen M, Tu H, Vuorela A, Notbohm H, Kivirikko KI, Myllyharju J (2001) High-level production of human type I collagen in the yeast Pichia pastoris. Yeast 18(9):797–806
Fratzl P, Weinkamer R (2007) Nature’s hierarchical materials. Prog Mater Sci 52(8):1263–1334
Leo L, Bridelli MG, Polverini E (2019) Insight on collagen self-assembly mechanisms by coupling molecular dynamics and UV spectroscopy techniques. Biophys Chem 253:106224
Liu X, Zheng C, Luo X, Wang X, Jiang H (2019) Recent advances of collagen-based biomaterials: multi-hierarchical structure, modification and biomedical applications. Mater Sci Eng C 99:1509–1522
Barthelat F, Yin Z, Buehler MJ (2016) Structure and mechanics of interfaces in biological materials. Nature Rev Mater 1:16007
Abas M, Masry ME, Elgharably H (2020) Collagen in diabetic wound healing. In: Wound healing, tissue repair, and regeneration in diabetes. Elsevier, Amsterdam, pp 393–401
Gu L, Shan T, Ma YX, Tay FR, Niu L (2019) Novel biomedical applications of crosslinked collagen. Trends Biotechnol 37(5):464–491
Carvalho AM, Marques AP, Silva TH, Reis RL (2018) Evaluation of the potential of collagen from codfish skin as a biomaterial for biomedical applications. Mar Drugs 16(12):495
Lee BA, Hudson AR, Shiwarski DJ, Tashman JW, Hinton TJ, Yerneni S, Bliley JM, Campbell PG, Feinberg AW (2019) 3D bioprinting of collagen to rebuild components of the human heart. Science 365:482–487
Zhu S, Yuan Q, Yin T, You J, Gu Z, Xiong S, Hu Y (2018) Self-assembly of collagen-based biomaterials: preparation, characterizations and biomedical applications. J Mater Chem B 6(18):2650–2676
Cisneros DA, Hung C, Franz CM, Muller DJ (2006) Observing growth steps of collagen self-assembly by time-lapse high-resolution atomic force microscopy. J Struct Biol 154(3):232–245
Yang W, Meyers MA, Ritchie RO (2019) Structural architectures with toughening mechanisms in nature: a review of the materials science of type-I collagenous materials. Prog Mater Sci 103(6):425–483
Vuorela A, Myllyharju J, Nissi R, Pihlajaniemi T, Kivirikko KI (1997) Assembly of human prolyl 4-hydroxylase and type III collagen in the yeast pichia pastoris: formation of a stable enzyme tetramer requires coexpression with collagen and assembly of a stable collagen requires coexpression with prolyl 4-hydroxylase. EMBO J 16(22):6702–6712
Vuorela A, Myllyharju J, Pihlajaniemi T, Kivirikko KI (1999) Coexpression with collagen markedly increases the half-life of the recombinant human prolyl 4-hydroxylase tetramer in the yeast Pichia pastoris. Matrix Biol 18(5):519–522
Myllyharju J, Lamberg A, Notbohm H, Fietzek PP, Pihlajaniemi T, Kivirikko KI (1997) Expression of wild-type and modified proα chains of human type I procollagen in insect cells leads to the formation of stable [α1(I)]2α2(I) collagen heterotrimers and [α1(I)]3 homotrimers but not [α2(I)]3 homotrimers. J Biol Chem 272(35):21824–21830
Acknowledgments
This work was supported by the National Natural Science Foundation of China [grant numbers. 51973116, U1832109, 21935002], the Users with Excellence Program of Hefei Science Center CAS [grant number 2019HSC-UE003], the starting grant of ShanghaiTech University, and State Key Laboratory for Modification of Chemical Fibers and Polymer Materials.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Science+Business Media, LLC, part of Springer Nature
About this protocol
Cite this protocol
Zhao, C., Xiao, Y., Ling, S., Pei, Y., Ren, J. (2021). Synthesis and Assembly of Recombinant Collagen. In: Ling, S. (eds) Fibrous Proteins. Methods in Molecular Biology, vol 2347. Humana, New York, NY. https://doi.org/10.1007/978-1-0716-1574-4_8
Download citation
DOI: https://doi.org/10.1007/978-1-0716-1574-4_8
Published:
Publisher Name: Humana, New York, NY
Print ISBN: 978-1-0716-1573-7
Online ISBN: 978-1-0716-1574-4
eBook Packages: Springer Protocols