Abstract
Genomic analyses increasingly make use of sophisticated statistical and computational approaches in investigations of genomic function and evolution. Scientists implementing and developing these approaches are often computational scientists, physicists, or mathematicians. This article aims to provide a compact overview of genome biology for these scientists. Thus, the article focuses on providing biological context to the genomic features, processes, and structures analysed by these approaches. Topics covered include (1) differences between eukaryotic and prokaryotic cells; (2) the physical structure of genomes and chromatin; (3) different categories of genomic regions, including those serving as templates for RNA and protein synthesis, regulatory regions, repetitive regions, and “architectural” or “organisational” regions, such as centromeres and telomeres; (4) the cell cycle; (5) an overview of transcription, translation, and protein structure; and (6) a glossary of relevant terms.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Budd, A. (2012) Diversity of genome organization. In Anisimova M., (ed.), Evolutionary genomics: statistical and computational methods (volume 1). Methods in Molecular Biology, Springer Science+Business media, LLC
Gerstein MB, Bruce C, Rozowsky JS, Zheng D, Du J, Korbel JO, Emanuelsson O, Zhang ZD, Weissman S, Snyder M (2007) What is a gene, post-ENCODE? History and updated definition. Genome Res 17:669–681
Goncalves Dos Santos Silva A, Sarkar R, Harizanova J, Guffei A, Mowat M, Garini Y, Mai S (2008) Centromeres in cell division, evolution, nuclear organization and disease. J Cell Biochem 104:2040–2058
Kapranov P (2009) From transcription start site to cell biology. Genome Biol 10:217
Alberts B, Johnson J, Lewis J, Raff M, Roberts K, Walter P (2007) Molecular Biology of the Cell 1392
Yakovchuk P, Protozanova E, Frank-Kamenetskii MD (2006) Base-stacking and base-pairing contributions into thermal stability of the DNA double helix. Nucleic Acids Res 34:564–574
Dineen DG, Wilm A, Cunningham P, Higgins DG (2009) High DNA melting temperature predicts transcription start site location in human and mouse. Nucleic Acids Res 37:7360–7367
Duffy S, Shackelton LA, Holmes EC (2008) Rates of evolutionary change in viruses: patterns and determinants. Nat Rev Genet 9:267–276
Charlesworth B (2009) Fundamental concepts in genetics: effective population size and patterns of molecular evolution and variation. Nat Rev Genet 10:195–205
Cairns BR (2009) The logic of chromatin architecture and remodelling at promoters. Nature 461:193–198
History of life through time UCMP http://www.ucmp.berkeley.edu/exhibits/historyoflife.php
Huber H, Hohn MJ, Stetter KO, Rachel R (2003) The phylum Nanoarchaeota: present knowledge and future perspectives of a unique form of life. Res Microbiol 154:165–171
Grant M, Mitton J (2010) Case Study: The glorious, golden, and gigantic quaking aspen Nature Educational Knowledge 1:40
Kuntner M, Agnarsson I (2006) Are the linnean and phylogenetic nomenclatural systems combinable? Recommendations for biological nomenclature. Syst Biol 55:774–784
Sapp J (2005) The prokaryote-eukaryote dichotomy: meanings and mythology. Microbiol Mol Biol Rev 69:292–305
Sapp J (2006) Two faces of the prokaryote concept. Int Microbiol 9:163–172
Whitman WB (2009) The modern concept of the procaryote. J Bacteriol 191:2000–5; discussion 2006–7
Pace NR (2006) Time for a change. Nature 441:289
Pace NR (2009) Problems with “procaryote”. J Bacteriol 191:2008–10; discussion 2011
Griffiths G (2007) Cell evolution and the problem of membrane topology. Nat Rev Mol Cell Biol 8:1018–1024
Szostak JW, Bartel DP, Luisi PL (2001) Synthesizing life. Nature 409:387–390
Platta HW, Erdmann R (2007) Peroxisomal dynamics. Trends Cell Biol 17:474–484
Tabak HF, Braakman I, Distel B (1999) Peroxisomes: simple in function but complex in maintenance. Trends Cell Biol 9:447–453
Trinkle-Mulcahy L, Lamond AI (2007) Toward a high-resolution view of nuclear dynamics. Science 318:1402–1407
Schneider R, Grosschedl R (2007) Dynamics and interplay of nuclear architecture, genome organization, and gene expression. Genes Dev 21:3027–3043
Logan DC (2006) The mitochondrial compartment. J Exp Bot 57:1225–1243
Saraste M (1999) Oxidative phosphorylation at the fin de siecle. Science 283:1488–1493
Lill R, Muhlenhoff U (2008) Maturation of iron-sulfur proteins in eukaryotes: mechanisms, connected processes, and diseases. Annu Rev Biochem 77:669–700
Lill R (2009) Function and biogenesis of iron-sulphur proteins. Nature 460:831–838
Dyall SD, Brown MT, Johnson PJ (2004) Ancient invasions: from endosymbionts to organelles. Science 304:253–257
Keeling PJ (2010) The endosymbiotic origin, diversification and fate of plastids. Philos Trans R Soc Lond B Biol Sci 365:729–748
Dillon SC, Dorman CJ (2010) Bacterial nucleoid-associated proteins, nucleoid structure and gene expression. Nat Rev Microbiol 8:185–195
Martinez-Antonio A, Medina-Rivera A, Collado-Vides J (2009) Structural and functional map of a bacterial nucleoid. Genome Biol 10:247
Thanbichler M, Wang SC, Shapiro L (2005) The bacterial® nucleoid: a highly organized and dynamic structure. J Cell Biochem 96:506–521
Witz G, Stasiak A (2010) DNA supercoiling and its role in DNA decatenation and unknotting. Nucleic Acids Res 38:2119–2133
Bock C, Lengauer T (2008) Computational epigenetics. Bioinformatics 24:1–10
Goldberg AD, Allis CD, Bernstein E (2007) Epigenetics: a landscape takes shape. Cell 128:635–638
Krueger AT, Kool ET (2007) Model systems for understanding DNA base pairing. Curr Opin Chem Biol 11:588–594
Watson JD, Crick FH (1953) Molecular structure of nucleic acids; a structure for deoxyribose nucleic acid. Nature 171:737–738
Olson WK, Esguerra M, Xin Y, Lu XJ (2009) New information content in RNA base pairing deduced from quantitative analysis of high-resolution structures. Methods 47:177–186
Ghosh A, Bansal M (2003) A glossary of DNA structures from A to Z. Acta Crystallogr D Biol Crystallogr 59:620–626
Potaman VN, Sinden RR. (2005) DNA: Alternative Confirmations and Biology, in DNA Confirmation and Transcription (Ohyama T, Ed.) pp 3–17, Springer
Oh DB, Kim YG, Rich A (2002) Z-DNA-binding proteins can act as potent effectors of gene expression in vivo. Proc Natl Acad Sci U S A 99:16666–16671
Voineagu I, Freudenreich CH, Mirkin SM (2009) Checkpoint responses to unusual structures formed by DNA repeats. Mol Carcinog 48:309–318
Mirkin SM (2006) DNA structures, repeat expansions and human hereditary disorders. Curr Opin Struct Biol 16:351–358
Goodsell DS (2001) The molecular perspective: ultraviolet light and pyrimidine dimers. Stem Cells 19:348–349
Muniandy PA, Liu J, Majumdar A, Liu ST, Seidman MM (2010) DNA interstrand crosslink repair in mammalian cells: step by step. Crit Rev Biochem Mol Biol 45:23–49
Gates KS (2009) An overview of chemical processes that damage cellular DNA: spontaneous hydrolysis, alkylation, and reactions with radicals. Chem Res Toxicol 22:1747–1760
Taylor EM, Lehmann AR (1998) Conservation of eukaryotic DNA repair mechanisms. Int J Radiat Biol 74:277–286
Clancy S (2008) DNA damage & repair: mechanisms for maintaining DNA integrity. Nature Education 1:B
Cedar H, Bergman Y (2009) Linking DNA methylation and histone modification: patterns and paradigms. Nat Rev Genet 10:295–304
Lister R, Ecker JR (2009) Finding the fifth base: genome-wide sequencing of cytosine methylation. Genome Res 19:959–966
Ooi SK, O’Donnell AH, Bestor TH (2009) Mammalian cytosine methylation at a glance. J Cell Sci 122:2787–2791
Borst P, Sabatini R (2008) Base J: discovery, biosynthesis, and possible functions. Annu Rev Microbiol 62:235–251
Maynard Smith J (1998) Evolutionary Genetics 354
Whitlock MC, Bürger R (2004) Fixation of new mutations in small populations. In Ferrieère R, Dieckmann U, Couvet D (eds.) Evolutionary Conservation Biology pp 155–170, Cambridge University Press
Elgar G, Vavouri T (2008) Tuning in to the signals: noncoding sequence conservation in vertebrate genomes. Trends Genet 24: 344–352
Luijsterburg MS, White MF, van Driel R, Dame RT (2008) The major architects of chromatin: architectural proteins in bacteria, archaea and eukaryotes. Crit Rev Biochem Mol Biol 43:393–418
Venters BJ, Pugh BF (2009) How eukaryotic genes are transcribed. Crit Rev Biochem Mol Biol 44:117–141
Johnson A, O’Donnell M (2005) Cellular DNA replicases: components and dynamics at the replication fork. Annu Rev Biochem 74:283–315
Sherratt DJ (2003) Bacterial chromosome dynamics. Science 301:780–785
Egan ES, Fogel MA, Waldor MK (2005) Divided genomes: negotiating the cell cycle in prokaryotes with multiple chromosomes. Mol Microbiol 56:1129–1138
Bernander R (2000) Chromosome replication, nucleoid segregation and cell division in archaea. Trends Microbiol 8:278–283
Ghosh SK, Hajra S, Paek A, Jayaram M (2006) Mechanisms for chromosome and plasmid segregation. Annu Rev Biochem 75:211–241
Margolin W (2000) Themes and variations in prokaryotic cell division. FEMS Microbiol Rev 24:531–548
Scholey JM, Brust-Mascher I, Mogilner A (2003) Cell division. Nature 422:746–752
Thanbichler M (2010) Synchronization of chromosome dynamics and cell division in bacteria. Cold Spring Harb Perspect Biol 2:a000331
Sclafani RA, Holzen TM (2007) Cell cycle regulation of DNA replication. Annu Rev Genet 41:237–280
Zakrzewska-Czerwinska J, Jakimowicz D, Zawilak-Pawlik A, Messer W (2007) Regulation of the initiation of chromosomal replication in bacteria. FEMS Microbiol Rev 31:378–387
Margulis L (2005) Hans Ris (1914–2004). Genophore, chromosomes and the bacterial origin of chloroplasts. Int Microbiol 8:145–148
Ris H, Kubai DF (1970) Chromosome structure. Annu Rev Genet 4:263–294
McHugh B, Heck MM (2003) Regulation of chromosome condensation and segregation. Curr Opin Genet Dev 13:185–190
Slater FR, Bailey MJ, Tett AJ, Turner SL (2008) Progress towards understanding the fate of plasmids in bacterial communities. FEMS Microbiol Ecol 66:3–13
Smillie C, Garcillan-Barcia MP, Francia MV, Rocha EP, de la Cruz F (2010) Mobility of plasmids. Microbiol Mol Biol Rev 74:434–452
Salje J (2010) Plasmid segregation: how to survive as an extra piece of DNA. Crit Rev Biochem Mol Biol 45:296–317
Hayes F (2003) The function and organization of plasmids. Methods Mol Biol 235: 1–17
Leplae R, Lima-Mendez G, Toussaint A (2006) A first global analysis of plasmid encoded proteins in the ACLAME database. FEMS Microbiol Rev 30:980–994
Vivian A, Murillo J, Jackson RW (2001) The roles of plasmids in phytopathogenic bacteria: mobile arsenals? Microbiology 147:763–780
Bhattacharya S, Som I, Bhattacharya A (1998) The ribosomal DNA plasmids of entamoeba. Parasitol Today 14:181–185
Farrar NA, Williams KL (1988) Nuclear plasmids in the simple eukaryotes Saccharomyces cerevisiae and Dictyostelium discoideum. Trends Genet 4:343–348
Griffiths AJ (1995) Natural plasmids of filamentous fungi. Microbiol Rev 59:673–685
Kutzler MA, Weiner DB (2008) DNA vaccines: ready for prime time? Nat Rev Genet 9:776–788
Hager GL, McNally JG, Misteli T (2009) Transcription dynamics. Mol Cell 35:741–753
Masai H, Matsumoto S, You Z, Yoshizawa-Sugata N, Oda M (2010) Eukaryotic chromosome DNA replication: where, when, and how? Annu Rev Biochem 79:89–130
Cramer P, Armache KJ, Baumli S, Benkert S, Brueckner F, Buchen C, Damsma GE, Dengl S, Geiger SR, Jasiak AJ, Jawhari A, Jennebach S, Kamenski T, Kettenberger H, Kuhn CD, Lehmann E, Leike K, Sydow JF, Vannini A (2008) Structure of eukaryotic RNA polymerases. Annu Rev Biophys 37:337–352
Aitken CE, Petrov A, Puglisi JD (2010) Single ribosome dynamics and the mechanism of translation. Annu Rev Biophys 39:491–513
Clancy S (2008) Genetic Recombination Nature Education 1:A
Heintzman ND, Ren B (2009) Finding distal regulatory elements in the human genome. Curr Opin Genet Dev 19:541–549
Maston GA, Evans SK, Green MR (2006) Transcriptional regulatory elements in the human genome. Annu Rev Genomics Hum Genet 7:29–59
Ghildiyal M, Zamore PD (2009) Small silencing RNAs: an expanding universe. Nat Rev Genet 10:94–108
Brierley I, Gilbert RJ, Pennell S (2008) RNA pseudoknots and the regulation of protein synthesis. Biochem Soc Trans 36:684–689
Baira E, Greshock J, Coukos G, Zhang L (2008) Ultraconserved elements: genomics, function and disease. RNA Biol 5:132–134
Licastro D, Gennarino VA, Petrera F, Sanges R, Banfi S, Stupka E (2010) Promiscuity of enhancer, coding and non-coding transcription functions in ultraconserved elements. BMC Genomics 11:151
Wang J, Lee AP, Kodzius R, Brenner S, Venkatesh B (2009) Large number of ultraconserved elements were already present in the jawed vertebrate ancestor. Mol Biol Evol 26:487–490
Richard GF, Kerrest A, Dujon B (2008) Comparative genomics and molecular dynamics of DNA repeats in eukaryotes. Microbiol Mol Biol Rev 72:686–727
Goodier JL, Kazazian HHJ (2008) Retrotransposons revisited: the restraint and rehabilitation of parasites. Cell 135:23–35
Vermaak D, Bayes JJ, Malik HS (2009) A surrogate approach to study the evolution of noncoding DNA elements that organize eukaryotic genomes. J Hered 100:624–636
O’Sullivan RJ, Karlseder J (2010) Telomeres: protecting chromosomes against genome instability. Nat Rev Mol Cell Biol 11:171–181
Smith DR, Hua J, Lee RW (2010) Evolution of linear mitochondrial DNA in three known lineages of Polytomella. Curr Genet 56:427–438
Buscaino A, Allshire R, Pidoux A (2010) Building centromeres: home sweet home or a nomadic existence? Curr Opin Genet Dev 20:118–126
Robinson NP, Bell SD (2005) Origins of DNA replication in the three domains of life. FEBS J 272:3757–3766
Duggin IG, Wake RG, Bell SD, Hill TM (2008) The replication fork trap and termination of chromosome replication. Mol Microbiol 70:1323–1333
Duret L, Galtier N (2009) Biased gene conversion and the evolution of mammalian genomic landscapes. Annu Rev Genomics Hum Genet 10:285–311
Eyre-Walker A, Hurst LD (2001) The evolution of isochores. Nat Rev Genet 2:549–555
Birney E, Stamatoyannopoulos JA, Dutta A, Guigo R, Gingeras TR, Margulies EH, Weng Z, Snyder M, Dermitzakis ET, Thurman RE, Kuehn MS, Taylor CM, Neph S, Koch CM, Asthana S et al. (2007) Identification and analysis of functional elements in 1% of the human genome by the ENCODE pilot project. Nature 447:799–816
Gerstein MB, Lu ZJ, Van Nostrand EL, Cheng C, Arshinoff BI, Liu T, Yip KY, Robilotto R, Rechtsteiner A, Ikegami K, Alves P, Chateigner A, Perry M, Morris M, Auerbach RK et al. (2010) Integrative analysis of the Caenorhabditis elegans genome by the modENCODE project. Science 330:1775–1787
Roy S, Ernst J, Kharchenko PV, Kheradpour P, Negre N, Eaton ML, Landolin JM, Bristow CA, Ma L, Lin MF, Washietl S, Arshinoff BI, Ay F, Meyer PE, Robine N et al. (2010) Identification of functional elements and regulatory circuits by Drosophila modENCODE. Science 330:1787–1797
van Bakel H, Nislow C, Blencowe BJ, Hughes TR (2010) Most “dark matter” transcripts are associated with known genes. PLoS Biol 8:e1000371
Tomilin NV (2008) Regulation of mammalian gene expression by retroelements and non-coding tandem repeats. Bioessays 30:338–348
Hasler J, Samuelsson T, Strub K (2007) Useful ‘junk’: Alu RNAs in the human transcriptome. Cell Mol Life Sci 64:1793–1800
Eickbush TH, Eickbush DG (2007) Finely orchestrated movements: evolution of the ribosomal RNA genes. Genetics 175:477–485
Evan GI, Vousden KH (2001) Proliferation, cell cycle and apoptosis in cancer. Nature 411:342–348
Budirahardja Y, Gonczy P (2009) Coupling the cell cycle to development. Development 136:2861–2872
Haeusser DP, Levin PA (2008) The great divide: coordinating cell cycle events during bacterial growth and division. Curr Opin Microbiol 11:94–99
Morgan DO (2006) The Cell Cycle: Principles of Control New Science Press, Ltd 327
Rampakakis E, Gkogkas C, Di Paola D, Zannis-Hadjopoulos M (2010) Replication initiation and DNA topology: The twisted life of the origin. J Cell Biochem 110:35–43
Barry ER, Bell SD (2006) DNA replication in the archaea. Microbiol Mol Biol Rev 70: 876–887
Errico A, Costanzo V (2010) Differences in the DNA replication of unicellular eukaryotes and metazoans: known unknowns. EMBO Rep 11:270–278
Mott ML, Berger JM (2007) DNA replication initiation: mechanisms and regulation in bacteria. Nat Rev Microbiol 5:343–354
Yao NY, O’Donnell M (2009) Replisome structure and conformational dynamics underlie fork progression past obstacles. Curr Opin Cell Biol 21:336–343
Dalgaard JZ, Eydmann T, Koulintchenko M, Sayrac S, Vengrova S, Yamada-Inagawa T (2009) Random and site-specific replication termination. Methods Mol Biol 521:35–53
Handel MA, Schimenti JC (2010) Genetics of mammalian meiosis: regulation, dynamics and impact on fertility. Nat Rev Genet 11:124–136
Storchova Z, Kuffer C (2008) The consequences of tetraploidy and aneuploidy. J Cell Sci 121:3859–3866
Groth A, Rocha W, Verreault A, Almouzni G (2007) Chromatin challenges during DNA replication and repair. Cell 128:721–733
Koster DA, Crut A, Shuman S, Bjornsti MA, Dekker NH (2010) Cellular strategies for regulating DNA supercoiling: a single-molecule perspective. Cell 142:519–530
Li B, Carey M, Workman JL (2007) The role of chromatin during transcription. Cell 128:707–719
Corpet A, Almouzni G (2009) Making copies of chromatin: the challenge of nucleosomal organization and epigenetic information. Trends Cell Biol 19:29–41
Misteli T, Soutoglou E (2009) The emerging role of nuclear architecture in DNA repair and genome maintenance. Nat Rev Mol Cell Biol 10:243–254
Hubner MR, Spector DL (2010) Chromatin dynamics. Annu Rev Biophys 39:471–489
Rando OJ, Chang HY (2009) Genome-wide views of chromatin structure. Annu Rev Biochem 78:245–271
Woodcock CL, Ghosh RP (2010) Chromatin higher-order structure and dynamics. Cold Spring Harb Perspect Biol 2:a000596
Cremer T, Cremer M (2010) Chromosome territories. Cold Spring Harb Perspect Biol 2:a003889
Lieberman-Aiden E, van Berkum NL, Williams L, Imakaev M, Ragoczy T, Telling A, Amit I, Lajoie BR, Sabo PJ, Dorschner MO, Sandstrom R, Bernstein B, Bender MA, Groudine M, Gnirke A, Stamatoyannopoulos J, Mirny LA, Lander ES, Dekker J (2009) Comprehensive mapping of long-range interactions reveals folding principles of the human genome. Science 326:289–293
Tamaru H (2010) Confining euchromatin/heterochromatin territory: jumonji crosses the line. Genes Dev 24:1465–1478
Kadauke S, Blobel GA (2009) Chromatin loops in gene regulation. Biochim Biophys Acta 1789:17–25
Clapier CR, Cairns BR (2009) The biology of chromatin remodeling complexes. Annu Rev Biochem 78:273–304
Misteli T (2007) Beyond the sequence: cellular organization of genome function. Cell 128:787–800
Naumova N, Dekker J (2010) Integrating one-dimensional and three-dimensional maps of genomes. J Cell Sci 123:1979–1988
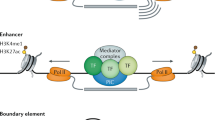
Visel A, Rubin EM, Pennacchio LA (2009) Genomic views of distant-acting enhancers. Nature 461:199–205
Ogbourne S, Antalis TM (1998) Transcriptional control and the role of silencers in transcriptional regulation in eukaryotes. Biochem J 331:1–14
Bushey AM, Dorman ER, Corces VG (2008) Chromatin insulators: regulatory mechanisms and epigenetic inheritance. Mol Cell 32:1–9
Li Q, Peterson KR, Fang X, Stamatoyannopoulos G (2002) Locus control regions. Blood 100:3077–3086
Goodrich JA, Tjian R (2010) Unexpected roles for core promoter recognition factors in cell-type-specific transcription and gene regulation. Nat Rev Genet 11:549–558
Smale ST, Kadonaga JT (2003) The RNA polymerase II core promoter. Annu Rev Biochem 72:449–479
Osbourn AE, Field B (2009) Operons. Cell Mol Life Sci 66:3755–3775
Russell J, Zomerdijk JC (2005) RNA-polymerase-I-directed rDNA transcription, life and works. Trends Biochem Sci 30:87–96
Sikorski TW, Buratowski S (2009) The basal initiation machinery: beyond the general transcription factors. Curr Opin Cell Biol 21:344–351
Carninci P, Sandelin A, Lenhard B, Katayama S, Shimokawa K, Ponjavic J, Semple CA, Taylor MS, Engstrom PG, Frith MC, Forrest AR, Alkema WB, Tan SL, Plessy C, Kodzius R, Ravasi T, Kasukawa T, Fukuda S, Kanamori-Katayama M, Kitazume Y, Kawaji H, Kai C, Nakamura M, Konno H, Nakano K, Mottagui-Tabar S, Arner P, Chesi A, Gustincich S, Persichetti F, Suzuki H, Grimmond SM, Wells CA, Orlando V, Wahlestedt C, Liu ET, Harbers M, Kawai J, Bajic VB, Hume DA, Hayashizaki Y (2006) Genome-wide analysis of mammalian promoter architecture and evolution. Nat Genet 38:626–635
Juven-Gershon T, Kadonaga JT (2010) Regulation of gene expression via the core promoter and the basal transcriptional machinery. Dev Biol 339:225–229
Buratowski S (2009) Progression through the RNA polymerase II CTD cycle. Mol Cell 36:541–546
Werner M, Thuriaux P, Soutourina J (2009) Structure-function analysis of RNA polymerases I and III. Curr Opin Struct Biol 19:740–745
Ciampi MS (2006) Rho-dependent terminators and transcription termination. Microbiology 152:2515–2528
Saunders A, Core LJ, Lis JT (2006) Breaking barriers to transcription elongation. Nat Rev Mol Cell Biol 7:557–567
Naville M, Gautheret D (2010) Transcription attenuation in bacteria: theme and variations. Brief Funct Genomics 9:178–189
Rougemaille M, Villa T, Gudipati RK, Libri D (2008) mRNA journey to the cytoplasm: attire required. Biol Cell 100:327–342
Cowling VH (2010) Regulation of mRNA cap methylation. Biochem J 425:295–302
Schellenberg MJ, Ritchie DB, MacMillan AM (2008) Pre-mRNA splicing: a complex picture in higher definition. Trends Biochem Sci 33:243–246
Millevoi S, Vagner S (2010) Molecular mechanisms of eukaryotic pre-mRNA 3′ end processing regulation. Nucleic Acids Res 38:2757–2774
Holste D, Ohler U (2008) Strategies for identifying RNA splicing regulatory motifs and predicting alternative splicing events. PLoS Comput Biol 4:e21
Wang Z, Burge CB (2008) Splicing regulation: from a parts list of regulatory elements to an integrated splicing code. RNA 14:802–813
Ram O, Ast G (2007) SR proteins: a foot on the exon before the transition from intron to exon definition. Trends Genet 23:5–7
Kornblihtt AR (2005) Promoter usage and alternative splicing. Curr Opin Cell Biol 17:262–268
Balagopal V, Parker R (2009) Polysomes, P bodies and stress granules: states and fates of eukaryotic mRNAs. Curr Opin Cell Biol 21:403–408
Lodish H, Berk A, Matsudaira P, Kaiser CA, Krieger M, Scott MP, Zipursky L, Darnell J. (2004) Section 1.2 The Molecules of Life, in Molecular Cell Biology Eds.) pp 8–13, W. H. Freeman, New York.
Rodnina MV, Wintermeyer W (2009) Recent mechanistic insights into eukaryotic ribosomes. Curr Opin Cell Biol 21:435–443
Schmeing TM, Ramakrishnan V (2009) What recent ribosome structures have revealed about the mechanism of translation. Nature 461:1234–1242
Wilkie GS, Dickson KS, Gray NK (2003) Regulation of mRNA translation by 5′- and 3′-UTR-binding factors. Trends Biochem Sci 28:182–188
Loh PG, Song H (2010) Structural and mechanistic insights into translation termination. Curr Opin Struct Biol 20:98–103
Ambrogelly A, Palioura S, Soll D (2007) Natural expansion of the genetic code. Nat Chem Biol 3:29–35
Agris PF (2004) Decoding the genome: a modified view. Nucleic Acids Res 32:223–238
Phizicky EM, Hopper AK (2010) tRNA biology charges to the front. Genes Dev 24:1832–1860
Ogle JM, Carter AP, Ramakrishnan V (2003) Insights into the decoding mechanism from recent ribosome structures. Trends Biochem Sci 28:259–266
Hopper AK, Phizicky EM (2003) tRNA transfers to the limelight. Genes Dev 17:162–180
Shoji S, Walker SE, Fredrick K (2009) Ribosomal translocation: one step closer to the molecular mechanism. ACS Chem Biol 4:93–107
Kochetov AV (2008) Alternative translation start sites and hidden coding potential of eukaryotic mRNAs. Bioessays 30:683–691
Petsko GA, Ringe D (2009) Chapter 1 From Sequence to Structure, in Protein Structure and Function (Primers in Biology) Eds.) pp 2–29, Oxford University Press
Parrott LM, Slater JH (1980) The DNA, RNA and protein composition of the cyanobacterium Anacystis nidulans grown in light- and carbon dioxide-limited chemostats. Arch Microbiol 127:53–58
Polakis ES, Bartley W (1966) Changes in dry weight, protein, deoxyribonucleic acid, ribonucleic acid and reserve and structural carbohydrate during the aerobic growth cycle of yeast. Biochem J 98:883–887
Katz U (1995) Cellular water content and volume regulation in animal cells. Cell Biochem Funct 13:189–193
Aliev MK, Dos Santos P, Hoerter JA, Soboll S, Tikhonov AN, Saks VA (2002) Water content and its intracellular distribution in intact and saline perfused rat hearts revisited. Cardiovasc Res 53:48–58
Warner JR (1999) The economics of ribosome biosynthesis in yeast. Trends Biochem Sci 24:437–440
Wilson DN, Nierhaus KH (2007) The weird and wonderful world of bacterial ribosome regulation. Crit Rev Biochem Mol Biol 42:187–219
Gutteridge A, Thornton JM (2005) Understanding nature’s catalytic toolkit. Trends Biochem Sci 30:622–629
Tokuriki N, Tawfik DS (2009) Protein dynamism and evolvability. Science 324:203–207
Branden C, Tooze J (1998) Introduction to Protein Structure 410
Marahiel MA (2009) Working outside the protein-synthesis rules: insights into non-ribosomal peptide synthesis. J Pept Sci 15:799–807
Lu Y, Freeland S (2006) On the evolution of the standard amino-acid alphabet. Genome Biol 7:102
Yuan J, O’Donoghue P, Ambrogelly A, Gundllapalli S, Sherrer RL, Palioura S, Simonovic M, Soll D (2010) Distinct genetic code expansion strategies for selenocysteine and pyrrolysine are reflected in different aminoacyl-tRNA formation systems. FEBS Lett 584:342–349
Farley AR, Link AJ (2009) Identification and quantification of protein posttranslational modifications. Methods Enzymol 463:725–763
Cloos PA, Christgau S (2002) Non-enzymatic covalent modifications of proteins: mechanisms, physiological consequences and clinical applications. Matrix Biol 21: 39–52
Young JC, Agashe VR, Siegers K, Hartl FU (2004) Pathways of chaperone-mediated protein folding in the cytosol. Nat Rev Mol Cell Biol 5:781–791
Tompa P (2010) Structure and Function of Intrinsically Disordered Proteins 331
Dyson HJ, Wright PE (2005) Intrinsically unstructured proteins and their functions. Nat Rev Mol Cell Biol 6:197–208
Ward JJ, Sodhi JS, McGuffin LJ, Buxton BF, Jones DT (2004) Prediction and functional analysis of native disorder in proteins from the three kingdoms of life. J Mol Biol 337:635–645
Voges D, Zwickl P, Baumeister W (1999) The 26S proteasome: a molecular machine designed for controlled proteolysis. Annu Rev Biochem 68:1015–1068
Peters JM (2006) The anaphase promoting complex/cyclosome: a machine designed to destroy. Nat Rev Mol Cell Biol 7:644–656
Nooren IM, Thornton JM (2003) Diversity of protein-protein interactions. EMBO J 22:3486–3492
Perkins JR, Diboun I, Dessailly BH, Lees JG, Orengo C (2010) Transient protein-protein interactions: structural, functional, and network properties. Structure 18:1233–1243
Olave IA, Reck-Peterson SL, Crabtree GR (2002) Nuclear actin and actin-related proteins in chromatin remodeling. Annu Rev Biochem 71:755–781
Carninci P (2010) RNA dust: where are the genes? DNA Res 17:51–59
Gardner PP, Daub J, Tate JG, Nawrocki EP, Kolbe DL, Lindgreen S, Wilkinson AC, Finn RD, Griffiths-Jones S, Eddy SR, Bateman A (2009) Rfam: updates to the RNA families database. Nucleic Acids Res 37:D136–40
Acknowledgements
Many thanks to Maria Anisimova, Sonia Furtado, Halldór Stefánsson, Nita Budd, and Damien Devos for many valuable comments and suggestions during the writing of this article.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Glossary
- 3′ End (“3′ terminus”)
-
One end of a polynucleotide molecule has a free (i.e. available to form additional chemical bonds with other atoms) hydroxyl (OH) functional group that is attached to the 3′ carbon atom of the sugar moiety of the terminal nucleotide. This is known as the 3′ “end” or “terminus” of the polynucleotide. Within the cell, the polynucleotides RNA and DNA can only be synthesised by attaching a nucleotide to a 3′ terminal hydroxyl functional group. Thus, the last nucleotide added to an RNA or DNA molecule is the 3′ terminal nucleotide. For this reason, RNA and DNA synthesis is described as taking place in a 5′- to 3′ direction. Therefore, the 3′ terminal nucleotide is sometimes referred to as the “last” nucleotide. Convention is to write RNA or DNA sequences ending with the 3′ terminal residue.
- 5′ Cap
-
A post-transcriptional modification of eukaryotic messenger RNAs (mRNAs), in which a methylated guanine nucleotide is added to the 5′ end of the mRNA.
- 5′ End (“5′ terminus”)
-
One end of a polynucleotide molecule has a free (i.e. available to form additional chemical bonds with other atoms) phosphate (PO4) moiety attached to the 5′ carbon atom of the sugar moiety of the terminal nucleotide. This is known as the 5′ “end” or “terminus” of the polynucleotide. Within the cell, the polynucleotides RNA and DNA can only be synthesised by attaching a nucleotide to a 3′ terminal hydroxyl group. Thus, the first nucleotide to be incorporated into an RNA or DNA molecule is the 5′ terminal nucleotide. For this reason, RNA and DNA synthesis is described as taking place in a 5′- to 3′ direction. Therefore, the 5′ terminal nucleotide is sometimes referred to as the “first” nucleotide. Convention is to write RNA or DNA sequences starting with the 5′ terminal residue.
- Alternative splicing
-
The incorporation of different sets of exons in different transcripts derived from the same gene. Genes that produce several different transcripts of this kind are described as being alternatively spliced. Alternative splicing is a feature of many human genes; it has been estimated that 95% of human genes that have more than one exon are alternatively spliced.
- Amino acid
-
A molecule consisting of a carboxylic acid (COOH) functional group, an amine (NH3) functional group, and a “side chain” moeity. Different amino acids have different side chains. Polypeptide chains are linear polymers of amino acids.
- Amino-terminus (“N-terminus”)
-
The end of a polypeptide chain with a free amine (NH2) functional group. Ribosomes synthesise polypeptides by attaching the amine group of an amino acid to the carboxyl group of a polypeptide. Thus, the first amino acid to be incorporated into a polypeptide chain is the amino-terminal residue. For this reason, polypeptides are described as being synthesised in an N-to-C direction and the amino-terminal residue is sometimes referred to as the “first” amino acid. Convention is to write polypeptide sequences beginning with the amino-terminal residue.
- Anti-codon
-
The region of a transfer RNA (tRNA) that specifically interacts with the corresponding codon in a messenger RNA during translation. Anticodons consist of three adjacent nucleotide residues within the tRNA sequence.
- Archaea
-
One of the two taxonomic groups into which all prokaryotes are divided, the other group being the bacteria.
- Adenosine triphosphate (ATP)
-
A nucleotide consisting of a ribose sugar moeity, an adenine nitrogenous base, and three-phosphate groups. The conversion of ATP to adenosine diphosphate (ADP) releases a phosphate group and a large amount of energy. This energy is used by the cell in a huge range of different processes. In this context, ATP acts as a “battery” of chemical energy for the cell.
- Autosome
-
A eukaryotic chromosome present in two nearly identical copies (homologous pairs) in diploid cells. This contrasts with sex chromosomes, where homologous pairs are typically rather different from each other in both sequence and structure.
- Backbone (DNA, RNA, or polypeptide)
-
The portion of a polymer that includes the moieties that lie along the line of direct links between individual monomers. The backbone is distinct from side group regions of the monomers. For DNA and RNA, monomers (nucleotides) are linked via bonds between phosphate and sugar groups; thus, DNA and RNA are described as having a sugar–phosphate backbone. In polypeptides, monomers (amino acids) are linked via peptide bonds; thus, proteins are described as having a peptide backbone.
- Bacteria
-
One of the two taxonomic groups into which all prokaryotes are divided, the other group being the archaea.
- Base (DNA or RNA)
-
Often used as shorthand for “nucleotide residue” in the context of DNA and RNA molecules. For example, the human genome is sometimes described as containing around 3 gigabases. This usage is based on the fact that nucleotide residues contain nitrogenous bases. More generally, in chemistry there are several different definitions of a base (the opposite of an acid).
- Biological membrane
-
A membrane consisting of a lipid bilayer, within which proteins are embedded, that partitions cellular contents into separate compartments. The cell membrane separates the entire cell from the environment; other biological membranes (those of membrane-bound organelles) separate regions of the cell into subcellular compartments. Different membranes contain different lipids (or different proportions of different proportions of different lipids) and different proteins.
- C-terminus (“carboxyl terminus”)
-
See “carboxyl terminus”.
- Canonical base pair (“complementary” or “Watson-Crick” base pair)
-
See “complementary base pair”.
- Capsid (viral)
-
The structure that packages the genome of a virus when it is outside its host cell. Capsids vary greatly in terms of the structure of the proteins and other components they consist of, and their overall shape.
- Carboxyl terminus (“C-terminus”)
-
One end of a polypeptide molecule has a free (i.e. available to form additional bonds with other atoms) carboxyl (COOH) functional group. The amino acid moiety containing this free carboxyl group is known as the carboxyl terminus (or carboxyl-terminal residue). Within the cell, polypeptides are synthesised by attaching an amino acid to a carboxyl terminus. Thus, the last amino acid to be incorporated into a polypeptide chain is the carboxyl-terminal residue. For this reason, polypeptides are described as being synthesised in an N-to-C direction and the carboxyl-terminal residue is sometimes referred to as the “last” amino acid. Convention is to write polypeptide sequences ending with the carboxyl-terminal residue.
- Cell cycle
-
A set of processes that interact to enable a cell to successfully divide into two daughter cells. The cycle involves replication of the cellular genome, cell division, and appropriate duplication and segregation of other cellular components prior to cell division. In eukaryotes, the cycle is divided into interphase (during which individual chromosomes cannot be distinguished by light microscopy) and either mitosis (for the majority of different cell types) or meiosis (for the production of gametes), where individual chromosomes are visible by light microscopy (such chromosomes are described as condensed). DNA replication occurs during S phase, a division of interphase. The region of interphase between cell division and S-phase is known as G1 (gap-1) phase, and that between S-phase and mitosis (or meiosis) as G2 (gap-2) phase. Mitosis and meiosis are also divided up into several different phases.
- Centromere
-
The region of a eukaryotic chromosome that is attached to the spindle apparatus during mitosis or meiosis. The spindle apparatus pulls chromosomes into a position where they can be successfully incorporated into a daughter cell resulting from cell division.
- Chain (polypeptide or protein)
-
One polypeptide molecule.
- Chromatid
-
One of the two copies of a eukaryotic chromosome produced following replication of the cellular genome during S phase of the cell cycle. “Sister” chromatids are copies of the same pre-S phase chromosome. During cell division, sister chromatids are separated so that one copy segregates into each of the daughter cells resulting from the cell division.
- Chromatin
-
Typically used to refer to the combination of protein, nucleic acids, and other cellular components that make up eukaryotic chromosomes. However, it is sometimes (rarely) used to refer to the compositionally rather different structures that make up prokaryotic chromosomes and plasmids.
- Chromosome
-
A structure consisting of a double-stranded molecule of DNA packaged and organised together with proteins and other cellular components (the combination of DNA, protein, and other components is referred to as chromatin). Sometimes, the word refers only to structures of this kind found in eukaryotic cells, the word “genophore” being used for such structures in prokaryotic cells. In other contexts, “chromosome” is used for such structures in both eukaryotic and prokaryotic cells.
- Chromosome condensation
-
During interphase, individual chromosomes cannot be distinguished by light microscopy, unlike in M-phase. The process by which diffuse interphase chromosomes change to the more defined condensed structures seen during M-phase is known as chromosome condensation.
- Chromosome territory
-
The region of the nucleus that a chromosome tends to occupy during interphase.
- Cis-acting regulatory element
-
A gene regulatory region that (1) is on the same chromosome as the gene and (2) provides binding sites for other components, such as transcription factors, whose presence and activity directly mediate the expression level of the gene.
- Coding gene
-
A gene whose primary product is a protein i.e. that is transcribed to yield messenger RNAs that can be translated by a ribosome to yield a protein.
- Codon
-
A set of three adjacent nucleotides in a messenger RNA (mRNA) molecule that specify an amino acid for incorporation into the polypeptide encoded by the mRNA. Not all sets of three nucleotides in an mRNA are a codon; nucleotides in the 5′ and 3′ UTRs are not part of any codon, nor are triplets of nucleotides in the coding region of the mRNA that overlap with codon boundaries.
- Complementary base pair (sometimes known as “canonical” or “Watson-Crick” base pair)
-
Pairs of nucleotide bases that form strong interactions via hydrogen bonds. In DNA, the two complementary base pairings are cytosine:guanine and adenine:thymine. The complementary pairs in RNA are the same except that uracil substitutes for thymine.
- Complementary sequence
-
Pairs of DNA (or RNA) molecules, with base sequences that allow formation of antiparallel dimers where all bases participate in complementary base pairing with the other molecule, are described as having complementary sequences.
- Complex (protein)
-
A structure consisting of two or more polypeptide chains, where the chains are linked by non-covalent bonds. Non-covalent interactions between proteins and nucleic acids are also referred to as complexes (for example RNA–protein complexes).
- Conserved nongenic sequence (also “ultraconserved element” or “ultraconserved region”)
-
See “ultraconserved element”.
- Cytosine methlyation
-
Attachment of a methyl (CH3) group to the base moiety of a cytosine nucleotide. In mammals, cytosine methylation can regulate levels of gene expression, typically resulting in a reduction or switching off of transcription from the regulated gene.
- Daughter cell
-
During cell division, a single parental (or mother) cell divides to yield two daughter cells.
- Diploid
-
A cell in which each chromosome found in a haploid cell of the same species is represented by a homologous pair of chromosomes.
- Domain (protein)
-
The term is used differently in different contexts. Structural biologists and bioinformaticians use “domain” to refer to a protein module that forms a stable, globular three-dimensional structure (or “fold”) in the cell, and that does not require interaction with other polypeptide chains to maintain this stable structure. However, cell and other biologists sometimes use the term more loosely, to refer to any subsequence of a polypeptide chain, typically (but not always) associated with a particular function.
- Downstream (within a nucleotide sequence)
-
Towards the 3′ terminus of a nucleotide strand. For example, the 3′ UTR of a messenger RNA (mRNA) lies towards the 3′ end of the mRNA molecule compared to the coding region of the mRNA.
- DNA (short for deoxyribonucleic acid)
-
The molecule that encodes the genomes of all cellular life on Earth. DNA is a polymer of nucleotides in which the sugar moiety of the nucleotide is 2-deoxyribose. The “backbone” of the molecule alternates between sugar and phosphate moieties. Pairs of DNA molecules with complementary sequences can form a right-handed antiparallel double-stranded helical structure, i.e. the famous “double helix”.
- Editing (RNA)
-
A process in which the sequence of bases in an RNA molecule is altered after the base has been incorporated into a transcript during the process of transcription. This can involve the insertion or deletion of nucleotides, or a change in the nitrogenous base attached to the sugar moiety of a nucleotide within the molecule.
- Epigenetic change
-
A change in gene function inherited by cellular offspring/daughter cells from their parental cell.
- Euchromatin
-
Weakly stained regions of chromatin as observed through a microscope under a range of different staining methods. Intensely stained regions are known as heterochromatin. Euchromatin tends to contain more genes and to have higher transcriptional activity than heterochromatin.
- Eukaryotic cell
-
Cells in which the majority of the genome is packaged within a nucleus. Typically, eukaryotic cells are larger and have a more complex internal organisation than prokaryotic cells.
- Exon
-
A region of a gene whose transcribed sequence is retained in an RNA molecule after splicing of the transcript has occurred.
- Expression (gene)
-
The process of producing a product (RNA or protein) from a gene, using the DNA sequence of the gene as a template for the sequence of the product molecule. For example, expression of a protein gene in a eukaryote involves, among others, the processes of transcription and translation (it may also involve splicing, polyadenylation, etc.)
- Functional group (chemical)
-
A group of atoms within an organic molecule that is responsible for characteristic chemical reactions of the molecule. See the entry for “group” in this glossary for a description of what is meant by that term in this context. “Functional group” is often used synonymously with the word “moiety”; however, these two words have distinct meanings, with moiety being used to generally describe groups within a molecule. Thus, a moiety can include several functional groups.
- Gamete
-
Eukaryotic cells that can fuse with other cells during fertilisation to produce a zygote; this is part of the process of sexual reproduction. Gametes are haploid, and combine to produce a diploid zygote.
- Gene
-
In most cases, a gene refers to a region of a genome that provides the information required to produce, and regulate the timing and level of production, of an RNA (in the case of a coding gene, also a polypeptide) molecule. Using this definition, a gene includes not just the region of the genome that serves as a template for the sequence of the RNA transcript produced from the gene, but also the regulatory regions that control the timing and level of production of the transcripts.
- Genetic code
-
A description of which amino acid (or the signal to stop translation) is encoded by each of the 64 different codons. Many amino acids are encoded by more than one codon; hence, the genetic code is described as redundant. Codons encoding the same amino acid are described as synonymous codons. Not all organisms use the same genetic code; indeed, the genetic code used by the genomes of endosymbiotic organelles (mitochondria and plastids) are different from those used in the nuclei of the cells containing the organelle.
- Genome
-
The complete set of heritable genetic information of a cell or organism.
- Group (of atoms within a molecule)
-
Refers to the use of the word in compound nouns, such as “functional group” or “chemical group” (not in the context of the periodic table, however). In this context, the word “group” typically is used to refer to a portion of a molecule within which all atoms form chemical bounds with at least one other atom within the group. One or more atoms within the group form chemical bonds with atoms that are not part of the group—as the group is only a portion of a larger molecule, and must therefore be connected to other atoms within the molecule by chemical bonds. If all bonds linking the group to the rest of the molecule were broken, then all atoms within the group would remain linked together (at least before the occurrence of any reactions that would act to change the structure of the group). Thus, a group is a distinct sub-structure of a complete molecule, within which atoms are linked by chemical bonds.
- Haploid
-
Describes a cell where each of the chromosomes in the genome is present in only a single copy. For example, nuclei in human haploid cells (for example, gametes, i.e. sperm or egg cells) contain 23 chromosomal DNA molecules (22 autosomes and one sex chromosome); in contrast, most human cells are diploid, and contain 46 chromosomal DNA molecules in their nucleus, with each chromosome present in the haploid cells represented by a homologous pair of chromosomes in the diploid cell.
- Heterochromatin
-
Intensely stained regions of chromatin as observed through a microscope under a range of different staining methods. Weakly stained regions are known as euchromatin. Heterochromatin tends to contain fewer genes and to have lower transcriptional activity than euchromatin.
- Homologous chromosome pair
-
A pair of similar chromosomes contained within the same diploid cell, where one member of the pair was inherited from each parent. The sequences and structures of homologous chromosomes are very similar; however, due to the occurrence of different mutations in the evolutionary history of the two members of pair, they almost certainly have (often only slightly) different sequences/structures.
- Interphase
-
The phase of the eukaryotic cell cycle in which individual chromosomes cannot be distinguished by light microscopy.
- Intron
-
A region of a gene that is transcribed, but where the region of the RNA transcript that was encoded by this region of the gene is removed via the process of splicing.
- Isochore
-
A region of a mammalian chromosome within which the proportions of the two different kinds of complementary base pairs are similar. The proportion of the different base pairs is often described in terms of the “CG content”, referring to the proportion of cytosine:guanine base pairs in the region.
- Lipid bilayer
-
A thin membrane that consists of a pair of lipid monolayers interacting via the hydrophobic tail regions of the lipids within them. Thus, a bilayer is approximately as thick as two lipid molecules.
- Lipid
-
A molecule that consists of a polar (and hence hydrophilic) head group and a non-polar (hydrophobic) carbohydrate tail region.
- M phase (“mitosis”)
-
See “mitosis”.
- Meiosis
-
Similar to mitosis, meiosis is a phase of the cell cycle in which eukaryotic chromosomes are segregated into sets to be inherited by daughter cells. The difference to mitosis is that, after two meiotic divisions (meiosis I and meiosis II), four haploid cells are produced from the initial diploid cell; mitosis produces instead two diploid daughter cells from one diploid parental cell.
- Membrane-bound organelle
-
A compartment of a cell that is surrounded by (at least one) lipid bilayer. Examples of organelles include nuclei and mitochondria.
- messenger RNA (mRNA)
-
RNA molecules produced via transcription of a coding gene that can be translated by a ribosome to yield a polypeptide chain. In eukaryotes, mRNAs may be modified by a range of different processes, including splicing, 5′ capping, and polyadenylation.
- Moiety
-
A group of atoms within a molecule. See the entry for “group” in this glossary for a description of what is meant by the term in this context. Often used synonymously with the term “functional group”—however, the term “moiety”, as defined by the International Union of Pure and Applied Chemistry (IUPAC), the principal international society of chemists, indicates that “moiety” can be used more generally than “functional groups”, to refer to any part of a molecule. Thus, a given moiety might contain several functional groups.
- Mitochondrion
-
Membrane-bound organelles found in almost all eukaryotic cells. In eukaryotes, they are the site of the oxidative phosphorylation metabolic pathway, which in many eukaryotes is an important source of ATP. Mitochondria also are the only site of synthesis for iron–sulphur clusters in eukaryotes. These clusters are necessary components of several essential eukaryotic proteins. Like plastids, mitochondria are derived from bacterial endosymbionts. The ancestral bacterium from which mitochondria are derived was present in the ancestor of all living eukaryotes. Mitochondria retain small portions of the genome of these bacterial ancestors.
- Mitosis (sometimes referred to as M-phase)
-
A phase of the cell cycle in which individual chromosomes/chromatids can be distinguished using light microscopy. During mitosis, sister chromatids are segregated so that each daughter cell resulting from cell division contains one copy of each pair of sister chromatids.
- Mutation
-
A change in the sequence of a genome.
- N-terminus (amino-terminus)
-
See “amino-terminus”.
- Non-coding gene
-
A gene whose primary product is an RNA molecule, rather than a polypeptide, i.e. that produces functional transcripts that are not translated by a ribosome to yield a polypeptide, for example transfer RNA genes.
- Non-synonymous (codon)
-
Codons are non-synonymous if they code for different amino acids.
- Nucleoid
-
The irregularly shaped, non-membrane-bound structure that contains the genome of a prokaryotic cell.
- Nucleosome
-
A complex of approximately 150 base pairs of genomic DNA wrapped around a core of histone proteins. DNA within a nucleosome is compacted into a smaller volume than it would occupy if it were not bound within a nucleosome.
- Nucleotide
-
Molecules consisting of several moieties: (1) a nitrogenous base (2) a five-carbon sugar (3) and between one and three phosphate groups. In DNA molecules, the nitrogenous bases are usually adenine (A), cytosine (C), guanine (G), or thymine (T). In RNA molecules, they are usually adenine (A), cytosine (C), guanine (G), or uracil (U). However, cellular DNA and RNA can also contain modified versions of these bases, such as 5-methylcytosine in DNA.
- Nucleus
-
A double-membrane bound organelle of eukaryotic cells that contains the majority of the cellular genome (the “nuclear genome”). However, in most cases, a eukaryotic cell also contains several organelles (i.e. mitochondria, and in some cases plastids) that also contain portions of the cellular genome (“organellar genomes”).
- Oligomer
-
A molecule consisting of several (typically up to 100) smaller units (monomers). Individual monomers within the oligomer have similar structures; for example, a nucleotide oligomer is made up of several nucleotide monomers covalently bound to each other. Molecules containing much larger numbers of monomeric units are referred to as polymers.
- Operon
-
A set of genes that are transcribed together as a single RNA transcript.
- Origin of replication
-
A region of genomic DNA at which DNA replication is initiated.
- Peptide
-
Two or more amino acids covalently bound via peptide bonds.
- Peptide bond
-
A covalent bond formed between a caboxyl (COOH) and an amino (NH2) functional group. During synthesis of the bond, a molecule of water is released.
- Peroxisome (sometimes called microbodies)
-
A membrane-bound organelle found in many eukaryotic cells. Peroxisomes are involved in many different processes, and separate the toxic products of some of these processes from the rest of the cell.
- Plasmid
-
Structures consisting of DNA molecules, proteins, and other cellular components. DNA components of plasmids can be either circular or linear although most plasmids encountered in molecular biology are circular. Many prokaryotes possess plasmids, as do some eukaryotes. The genes within plasmids tend to be associated with functions that promote or enable survival and growth under specific “niche” conditions. They can be horizontally transferred between cells (i.e. not inherited from a parental cell as a result of cell division), and are typically replicated independently of the cell cycle, unlike chromosomes.
- Plastid
-
Membrane-bound organelles found in plants and some other eukaryotic organisms, participating in a range of different processes within the cell. Like mitochondria, plastids are derived from an endosymbiosis with a bacterium. Plastids retain remnants of their ancestral bacterial genome.
- Ploidy
-
A description of how many different homologous copies there are of each chromosome within a genome.
- Polyadenylation
-
The process by which several additional adenine-containing nucleotides are attached to the 3′ end of a transcript. Not all transcripts are polyadenylated.
- Polymer
-
A molecule consisting of many smaller units (monomers). Monomers are typically connected to each other via covalent chemical bonds. In this context, “many” is not strictly defined. However, molecules containing between 2 and 100 monomeric units are often referred to as oligomers.
- Polymerase (DNA or RNA)
-
An enzyme that polymerises the synthesis of polynucleotide chains from nucleotide molecules. DNA replication is mediated by a DNA polymerase, transcription by an RNA polymerase.
- Polynucleotide
-
A polymer of nucleotides. Both DNA and RNA are polynucleotides.
- Polypeptide
-
A linear polymer of amino acids bound together by covalent peptide bonds. Polypeptides are synthesised by ribosomes via the process of translation, using the nucleotide base sequence of a messenger RNA molecule as a template for the amino acid sequence of the polypeptide.
- Post-transcriptional modification
-
Changes made to the structure of an RNA molecule following transcription. Many different post-transcriptional modifications have been identified, some of which are essential for the cell. For example, the addition of an activated amino acid to the 3′ end of transfer RNAs (tRNAs) is essential for the process of translation.
- Prokaryotic cell
-
Cells within which the genome is not separated from the rest of a cell by a nucleus. Generally, prokaryotic cells are considerably smaller, and have a less complex internal organisation, than eukaryotic cells. The two most general taxonomic groupings within the prokaryotes are the archaea and the bacteria.
- Promoter
-
A regulatory region of a gene located close to its target gene, i.e. the gene (or genes) whose transcriptional activity it regulates. The protein complexes responsible for initiating transcription bind to regions of the promoter.
- Protein
-
Molecules (molecular complexes, if the protein contains more than one polypeptide) that consist of one or more polypeptide chains, and sometimes also additional non-polypeptide components. For example, a functional haemoglobin protein consists of four polypeptide chains, each of which is also bound to a non-polypeptide haeme molecule.
- Protein module
-
Regions of protein sequence (i.e. sub-sequences of polypeptide sequences) that mediate important aspects of their function independently of other regions of the full polypeptide chain. Protein domains and linear motifs are examples of protein modules.
- Proteasome
-
A protein complex found in all eukaryotes and archaea, and some bacteria, that is responsible for breaking down proteins into small peptides of approximately eight amino acids. Damaged proteins, and proteins that need to be degraded as part of cellular processes, are targeted to the proteasome.
- Recombination
-
A process in which genetic material is exchanged between different chromosomes, or between regions of the same chromosome.
- Remodelling enzyme (chromatin)
-
An enzyme that changes chromatin structure by repositioning, removing or assembling nucleosomes.
- Repeat (DNA)
-
Regions of DNA sequence within the same genome that are very similar/identical to each other.
- Replication (DNA)
-
The process of duplicating a DNA molecule to yield two copies with the same sequence as the original molecule. In practice, due to errors introduced during the process of replication, the two copies of the initial DNA molecule may have slightly different sequences.
- Residue (usually protein, sometimes also DNA and RNA)
-
Typically used to refer to amino acid moieties within a peptide. Within a peptide, amino acids are linked by peptide bonds. Formation of these bonds is accompanied by the loss of a water molecule (a hydrogen atom from the amino group of one amino acid combining with a hydroxyl (OH) group from the carboxyl group of the other amino acid). Thus, the amino acid monomers incorporated in the peptide are the remnants (or the “residue”) left behind after the loss of this water molecule. The linking of nucleotides via phosphodiester bonds, as occurs in the backbone of RNA and DNA molecules, also releases water; thus, individual nucleotides within these molecules are also sometimes referred to as “residues”.
- Ribonucleic acid (RNA)
-
A polymer of nucleotides in which the sugar moiety of the nucleotide is ribose. Plays an essential role in many cellular processes, including transcription, translation, and replication.
- ribosomal RNA (rRNA)
-
RNA molecules that are essential components of all ribosomes, both structurally and via direct involvement in catalysing the synthesis of polypeptide chains during the process of translation.
- Ribosome
-
The complex of proteins and RNA molecules that uses messenger RNA as a template for the synthesis of polypeptide chains via the process of translation.
- S phase
-
The phase of the eukaryotic cell cycle in which the genome is replicated.
- Sex chromosome
-
Some eukaryotes, such as mammals, use differences between particular pairs of chromosomes (the sex chromosomes) to determine the gender of an organism. In humans, there are two sex chromosomes, X and Y; they have very different lengths, and only a small region of the considerably larger X chromosome shares extensive sequence similarity with the Y chromosome. However, despite these differences, they are still sometimes considered a homologous pair of chromosomes, as they pair together at the metaphase plate during meiosis I.
- Sister chromatids
-
See “chromatid”.
- Splicing
-
The process by which regions (introns) of the initial transcript of a gene are removed, retaining only the exons.
- Strand (DNA)
-
One of the two DNA molecules within a double-stranded DNA molecule is often referred to as a strand.
- Small molecule
-
A term used by biochemists and pharmacologists to refer to non-polymer organic molecules. For example, individual amino acids, nucleotides, simple sugars, and many drugs are described as small molecules.
- Supercoiling (DNA)
-
Wrapping of the DNA double helix around itself. Supercoiling yields a more compact structure compared to DNA in a relaxed state. Supercoils can be induced by changing the number of times the two strands of a DNA double helix wrap around each other compared to in their relaxed state.
- Synonymous (codon)
-
Codons are synonymous if they code for the same amino acid.
- Taxonomic group (taxon)
-
Previously, this term was used to describe groups of organisms that share similar characteristics; current usage is to apply it, if possible, only to such groups where the organisms in the group are believed to represent all the descendents of a single common ancestor. Taxonomic groups are organised hierarchically; within more general groups, organisms are further classified into more specialised sub-groups. For example, the more general group Eukarya (eukaryotes) includes, among others, the groups Animalia (animals) and Plantae (plants); humans are members of both Eukarya and Animalia, but not Plantae.
- Telomere
-
Structures located at the ends of linear chromosomes or plasmids. Often “telomere” refers only to such structures in eukaryotic cells; however, it is also sometimes used to refer to such structures in both eukaryotes and prokaryotes.
- Topoisomerase
-
An enzyme that changes the number of times the two DNA strands within a DNA double helix twist around each other. This can act to introduce or relax supercoils in DNA molecules.
- Transcript
-
An RNA molecule synthesised via the process of transcription.
- Transcription
-
The process of synthesising an RNA molecule (a transcript) using the sequence of nucleotide bases in the DNA sequence as a template for the sequence of nucleotide bases in the transcript.
- Transcription factor
-
A protein that binds to a region of a chromosome or plasmid, as a result activating or repressing the expression of a gene.
- Transcription start site
-
The nucleotide position in a DNA sequence corresponding to the first base of an RNA transcript.
- Transfer RNA (tRNA)
-
Essential components of the translation apparatus that provide a physical link between the codons in a messenger RNA (mRNA) sequence and the amino acids coded for by the mRNA. Interaction with a specific codon in the mRNA is mediated via an anticodon within the tRNA molecule. Prior to participating in translation, the amino acid corresponding to the codon recognised by the tRNA is covalently attached to the 3′ terminus of the RNA molecule. During translation, this amino acid is incorporated in the polypeptide chain encoded by the mRNA.
- Translation
-
The process of synthesising a polypeptide molecule using the coding region of a messenger RNA (mRNA) molecule as a template. Translation is mediated by the ribosome.
- Trinucleotide repeat
-
A region of a nucleotide polymer that contain two or more adjacent copies of a given sequence of three nucleotide bases.
- Ultraconserved element or UCE (also “conserved nongenic sequence (CNS)” or “ultraconserved region (UCR)”)
-
Regions of non-coding sequence that are very strongly conserved between different organisms. Evidence suggests that these regions are functional, in some cases as regulatory sequences. However, in most cases the function of such elements is unknown.
- Ultraconserved region or UCR (also “conserved nongenic sequence” (CNS) or “ultraconserved element” (UCE))
-
See “ultraconserved element”.
- Untranslated region (UTR)
-
Regions of a messenger RNA (mRNA) molecule that do not encode the amino acid sequence of a polypeptide i.e. that do not overlap with any codons. Each mRNA has two UTRs, one on either side of the coding regions; the 3′ and 5′ UTRs.
- Upstream (within a nucleotide sequence)
-
Towards the 5′ terminus of a nucleotide strand. For example, the 5′ UTR of a messenger RNA (mRNA) lies towards the 5′ end of the mRNA molecule compared to the coding region of the mRNA.
- Untranslated region (UTR)
-
See “untranslated region”.
- Watson–Crick base pair (also known as “complementary” or “canonical” base pairs)
-
See “complementary base pair”.
- Zygote
-
A cell formed through the fusion of two gametes during the process of sexual reproduction. The zygote combines the haploid genomic material of the two gametes within one diploid cell (the zygote).
Rights and permissions
Copyright information
© 2012 Springer Science+Business Media, LLC
About this protocol
Cite this protocol
Budd, A. (2012). Introduction to Genome Biology: Features, Processes, and Structures. In: Anisimova, M. (eds) Evolutionary Genomics. Methods in Molecular Biology, vol 855. Humana Press, Totowa, NJ. https://doi.org/10.1007/978-1-61779-582-4_1
Download citation
DOI: https://doi.org/10.1007/978-1-61779-582-4_1
Published:
Publisher Name: Humana Press, Totowa, NJ
Print ISBN: 978-1-61779-581-7
Online ISBN: 978-1-61779-582-4
eBook Packages: Springer Protocols