Abstract
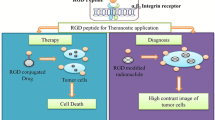
Active targeting has been explored for improving accumulation of drugs at the tumor site via specific ligand receptor interactions. The tripeptide “Arg-Gly-Asp” or RGD has shown tremendous potential as a targeting ligand in improving the delivery of drugs and diagnostic agents to integrin-overexpressing tumors. The different integrin-based targeting drug delivery systems studied include polymeric nanoparticles, polymeric micelles, and dendrimers, most of which are prepared by decorating RGD ligand on the surface of the drug delivery system. Our group previously reported the potential of peptide-based amphiphiles for integrin targeting of hydrophobic drugs. These amphiphiles are built by solid-phase peptide synthesis and contain RGD as the hydrophilic head group (also as a targeting ligand), a fatty acid as lipid tail and multiple units of hydrophilic linker. The focus of this chapter is to outline methodologies used for the synthesis, characterization, and evaluation of these low-molecular-weight RGD-based micellar carriers for delivery of hydrophobic anticancer agents. The experimental details and factors to be considered for optimal methods are discussed.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Byrne J, Betancourt T, Peppas L (2008) Active targeting schemes for nanoparticle systems in cancer therapeutics. Adv Drug Deliv Rev 60:1615–1626
Ramsay A, Marshall J, Hart I (2007) Integrin trafficking and its role in cancer metastasis. Cancer Metastasis Rev 26(3-4):567–578
Xiong J, Stehle T, Zhang R et al (2002) Crystal structure of the extracellular segment of integrin αvβ3 in complex with an Arg-Gly-Asp ligand. Science 296(5565):151–155
Allman R, Cowburn P, Mason M (2000) In vitro and in vivo effects of a cyclic peptide with affinity for the αvβ3 integrin in human melanoma cells. Eur J Cancer 36(3):410–422
Reardon D, Nabors L, Stupp R et al (2008) Cilengitide: an integrin-targeting arginine–glycine–aspartic acid peptide with promising activity for glioblastoma multiforme. Expert Opin Investig Drug 17(8):1225–1235
Mas-Moruno C, Rechenmacher F, Kessler H (2010) Cilengitide: the first anti-angiogenic small molecule drug candidate. Design, synthesis and clinical evaluation. Anticancer Agents Med Chem 10(10):753–768
Hartgerink J, Beniash E, Stupp S (2002) Peptide-amphiphile nanofibers: a versatile scaffold for the preparation of self-assembling materials. Proc Natl Acad Sci U S A 99(8):5133–5138
Wang Y, Wang X, Zhang Y et al (2009) RGD modified polymeric micelles as potential carriers for targeted delivery to integrin overexpressing tumor vasculature and tumor cells. J Drug Target 17(6):459–467
Danhier F, Vroman B, Lecouturier N et al (2009) Targeting of tumor endothelium by RGD-grafted PLGA-nanoparticles loaded with Paclitaxel. J Control Release 140(2):166–173
Kotamraj P, Russu W, Jasti B et al (2011) Novel integrin-targeted binding triggered drug delivery system for methotrexate. Pharm Res 28(12):3208–3219
Holig P, Bach M, Volkel T et al (2004) Novel RGD lipopeptides for the targeting of liposomes to integrin expressing endothelial and melanoma cells. Protein Eng Des Sel 17(5):433–441
Shukla R, Thomas T, Peters J (2005) Tumor angiogenic vasculature targeting with PAMAM dendrimer–RGD conjugates. Chem Commun (Camb) 46:5739–5741
Javali N, Raj A, Saraf P et al (2012) Fatty acid -RGD peptide amphiphile micelles as potential paclitaxel delivery carriers to αvβ3 integrin overexpressing tumors. Pharm Res 29(12):3347–3361
Raj A, Saraf P, Javali N et al (2014) Binding and uptake of novel RGD micelles to the αvβ3 integrin receptor for targeted drug delivery. J Drug Target 22(6):518–527
Saraf P, Li X, Wrischnik L et al (2015) In vitro and in vivo efficacy of self-assembling RGD peptide amphiphiles for targeted delivery of paclitaxel. Pharm Res 32(9):3087–3101
Domínguez A, Fernández A, González N et al (1997) Determination of critical micelle concentration of some surfactants by three techniques. J Chem Educ 74(10):1227–1231
Ray G, Chakraborty I, Moulik S (2006) Pyrene absorption can be a convenient method for probing critical micellar concentration (cmc) and indexing micellar polarity. J Colloid Interface Sci 294(1):248–254
Leng Y (2009) Materials characterization: introduction to microscopic and spectroscopic methods. Wiley, New York, NY. ISBN 978-0-470-82299-9
Han S, Cao S, Wang Y et al (2011) Self-Assembly of short peptide amphiphiles: the cooperative effect of hydrophobic interaction and hydrogen bonding. Chemistry 17(46):13095–13102
Lu J, Owen S, Stoichet M (2011) Stability of self-assembled polymeric micelles in serum. Macromolecules 44(15):6002–6008
Chen H, Kim S, Wang S et al (2008) Release of hydrophobic molecules from polymer micelles into cell membranes revealed by Förster resonance energy transfer imaging. Proc Natl Acad Sci U S A 105(18):6596–6601
Dong H, Dube N, Shu J et al (2012) Long circulating 15 nm micelles based on amphiphilic 3-Helix Peptide-PEG Conjugates. ACS Nano 6(6):5320–5329
Moerke N (2009) Fluorescence polarization (FP) assays for monitoring peptide-protein or nucleic acid–protein binding. Curr Protoc Chem Biol 1(1):1–15
Wang W, Wu Q, Pasuelo M et al (2005) Probing for integrin αvβ3 binding of RGD peptides using fluorescence polarization. Bioconjug Chem 16(3):729–734
Welsh D, Smith D (2011) Comparing dendritic and self-assembly strategies to multivalency-RGD peptide-integrin interactions. Org Biomol Chem 9(13):4795–4801
Vorup-Jensen T (2012) Surface plasmon resonance biosensing in studies of the binding between β2 integrin I domains and their ligands. Methods Mol Biol 757:55–71
Price R, Jerome W (eds) (2011) Basic confocal microscopy. Springer, New York, NY
Vega-Avila E, Pugsley M (2011) An overview of colorimetric assay methods used to assess survival or proliferation of mammalian cells. Proc West Pharmacol Soc 54:10–14
Vichai V, Kirtikara K (2006) Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat Protoc 1(3):1112–1116
Sylvester P (2011) Optimization of the tetrazolium dye (MTT) colorimetric assay for cellular growth and viability. Methods Mol Biol 716:157–168
Amblard M, Fehrentz J, Martinez J et al (2006) Methods and protocols of modern solid phase peptide synthesis. Mol Biotechnol 33(3):239–254
Coin I, Beyermann M, Bienert M (2007) Solid-phase peptide synthesis: from standard procedures to the synthesis of difficult sequences. Nat Protoc 2(12):3247–3256
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer Science+Business Media New York
About this protocol
Cite this protocol
Saraf, P., Li, X., Jasti, B. (2015). Integrin Targeting Using RGD-Based Peptide Amphiphiles. In: Patsenker, E. (eds) Integrin Targeting Systems for Tumor Diagnosis and Therapy. Methods in Pharmacology and Toxicology. Humana Press, New York, NY. https://doi.org/10.1007/7653_2015_61
Download citation
DOI: https://doi.org/10.1007/7653_2015_61
Published:
Publisher Name: Humana Press, New York, NY
Print ISBN: 978-1-4939-7443-6
Online ISBN: 978-1-4939-7445-0
eBook Packages: Springer Protocols