Abstract
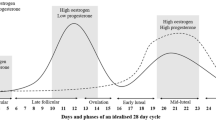
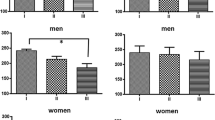
Background: In females, estrogen is a potential modulator of cortisol response to stressors. The aim of this study was to determine the influence of menstrual cycle phase, oral contraception (OC) use and exercise training on hypothalamo-pituitary-adrenal (HPA) axis activity and reactivity after physical stress. Aim: We investigated the effects of the menstrual cycle and OC use on exhaustive exercise-induced changes in free salivary cortisol concentrations and free urinary cortisol/cortisone excretion in healthy young women. Materials and subjects: Twenty-eight women were allocated to an untrained group (no.=16) or a trained group (no.=12), depending on their physical training background. The untrained group was composed of nine OC users (UNT-OC+) and seven eumenorrheic women (UNT-OC−) tested in the follicular and luteal phases, while the trained group was entirely composed of OC+ subjects (T-OC+). Methods: Three laboratory sessions were conducted in a randomised order: a prolonged exercise test, a short-term exercise test, and a control session. For each session, urine and saliva specimens were collected at rest (09: 00 h) and then, 30, 60 and 90 min later. Results: Estradiol fluctuation during the menstrual cycle phase did not alter free cortisol baseline values and responses to exercise. OC use was associated with increased free resting salivary concentrations and urinary cortisol excretion with blunted salivary cortisol response to prolonged exercise stimulation. No training effect was noted. Conclusions: OC but not menstrual cycle phase is associated with increased free cortisol levels and low HPA axis reactivity.
Similar content being viewed by others
References
Edwards KM, Mills PJ. Effects of estrogen versus estrogen and progesterone on cortisol and interleukin-6. Maturitas 2008, 61: 330–3.
Cucinelli F, Soranna L, Barini A, et al. Estrogen treatment and body fat distribution are involved in corticotropin and cortisol response to corticotropin-releasing hormone in postmenopausal women. Metabolism 2002, 51: 137–43.
Roca CA, Schmidt PJ, Altemus M, et al. Differential menstrual cycle regulation of hypothalamic-pituitary-adrenal axis in women with premenstrual syndrome and controls. J Clin Endocrinol Metab 2003, 88: 3057–63.
Kirschbaum C, Kudielka BM, Gaab J, Schommer NC, Hellhammer DH. Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus-pituitary-adrenal axis. Psychosom Med 1999, 61: 154–62.
Collins A, Eneroth P, Landgren BM. Psychoneuroendocrine stress responses and mood as related to the menstrual cycle. Psychosom Med 1985, 47: 512–27.
Bonen A, Haynes FJ, Watson-Wright W, et al. Effects of menstrual cycle on metabolic responses to exercise. J Appl Physiol 1983, 55: 1506–13.
Galliven EA, Singh A, Michelson D, Bina S, Gold PW, Deuster PA. Hormonal and metabolic responses to exercise across time of day and menstrual cycle phase. J Appl Physiol 1997, 83: 1822–31.
Kanaley JA, Boileau RA, Bahr JM, Misner JE, Nelson RA. Cortisol levels during prolonged exercise: the influence of menstrual phase and menstrual status. Int J Sports Med 1992, 13: 332–6.
Kirschbaum C, Platte P, Pirke KM, Hellhammer DH. Adrenocortical activation following stressful exercise: further evidence for attenuated free cortisol responses in women using oral contraceptives. Stress Med 1996, 12: 137–43.
Perogamvros I, Aarons L, Miller AG, Trainer PJ, Ray DW. Corticosteroid-binding globulin regulates cortisol pharmacokinetics. Clin Endocrinol (Oxf) 2011, 74: 30–6.
Bright GM, Darmaun D. Corticosteroid-binding globulin modulates cortisol concentration responses to a given production rate. J Clin Endocrinol Metab 1995, 80: 764–9.
Laudat MH, Cerdas S, Fournier C, Guiban D, Guilhaume B, Luton JP. Salivary cortisol measurement: a practical approach to assess pituitary-adrenal function. J Clin Endocrinol Metab 1988, 66: 343–8.
Rohleder N, Wolf JM, Piel M, Kirschbaum C. Impact of oral contraceptive use on glucocorticoid sensitivity of pro-inflammatory cytokine production after psychosocial stress. Psychoneuroendocrinology 2003, 28: 261–73.
Fries E, Hesse J, Hellhammer J, Hellhammer DH. A new view on hypocortisolism. Psychoneuroendocrinology 2005, 30: 1010–6.
Chrousos GP. Stress and disorders of the stress system. Nat Rev Endocrinol 2009, 5: 374–81.
Seckl JR, Walker BR. Minireview: 11 beta-hydroxysteroid dehydrogenase type 1 — a tissue-specific amplifier of glucocorticoid action. Endocrinology 2001, 142: 1371–6.
Hughes KA, Manolopoulos KN, Iqbal J, et al. Recycling between cortisol and cortisone in human splanchnic, subcutaneous adipose, and skeletal muscle tissues in vivo. Diabetes 2012, 61: 1357–64.
Buono MJ, Yeager JE, Sucec AA. Effect of aerobic training on the plasma ACTH response to exercise. J Appl Physiol 1987, 63: 2499–501.
Luger A, Deuster PA, Kyle SB, et al. Acute hypothalamic-pituitaryadrenal responses to the stress of treadmill exercise. Physiologic adaptations to physical training. N Engl J Med 1987, 316: 1309–15.
Wittert G. The effect of exercise on the hypothalamo-pituitaryadrenal-axis. In: Warren MP, Costantini NW (eds). Sports Endocrinology. Totowa, New Jersey: Humana Press; 2000.
Duclos M, Corcuff JB, Pehourcq F, Tabarin A. Decreased pituitary sensitivity to glucocorticoids in endurance-trained men. Eur J Endocrinol 2001, 144: 363–8.
Duclos M, Corcuff JB, Rashedi M, Fougere V, Manier G. Trained versus untrained men: different immediate post-exercise responses of pituitary adrenal axis. A preliminary study. Eur J Appl Physiol Occup Physiol 1997, 75: 343–50.
Gouarne C, Groussard C, Gratas-Delamarche A, Delamarche P, Duclos M. Overnight urinary cortisol and cortisone add new insights into adaptation to training. Med Sci Sports Exerc 2005, 37: 1157–67.
Vandewalle H, Peres G, Heller J, Monod H. All out anaerobic capacity tests on cycle ergometers. A comparative study on men and women. Eur J Appl Physiol Occup Physiol 1985, 54: 222–9.
Durnin JV, Rahaman MM. The assessment of the amount of fat in the human body from measurements of skinfold thickness. Br J Nutr 1967, 21: 681–9.
Enea C, Boisseau N, Ottavy M, et al. Effects of menstrual cycle, oral contraception, and training on exercise-induced changes in circulating DHEA-sulphate and testosterone in young women. Eur J Appl Physiol 2009, 106: 365–73.
Hay M, Mormede P. Improved determination of urinary cortisol and cortisone, or corticosterone and 11-dehydrocorticosterone by high-performance liquid chromatography with ultraviolet absorbance detection. J Chromatogr B Biomed Sci Appl 1997, 702: 33–9.
Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. New York: Routledge Academic. 1988.
De Souza MJ, Maguire MS, Maresh CM, Kraemer WJ, Rubin KR, Loucks AB. Adrenal activation and the prolactin response to exercise in eumenorrheic and amenorrheic runners. J Appl Physiol 1991, 70: 2378–87.
Timon R, Corvillo M, Brazo J, Robles MC, Maynar M. Strength training effects on urinary steroid profile across the menstrual cycle in healthy women. Eur J Appl Physiol 2013, 113: 1469–75.
Copeland JL, Consitt LA, Tremblay MS. Hormonal responses to endurance and resistance exercise in females aged 19–69 years. J Gerontol A Biol Sci Med Sci 2002, 57: B158–65.
Lin CL, Wu TJ, Machacek DA, Jiang NS, Kao PC. Urinary free cortisol and cortisone determined by high performance liquid chromatography in the diagnosis of Cushing’s syndrome. J Clin Endocrinol Metab 1997, 82: 151–5.
Kirschbaum C, Pirke KM, Hellhammer DH. Preliminary evidence for reduced cortisol responsivity to psychological stress in women using oral contraceptive medication. Psychoneuroendocrinology 1995, 20: 509–14.
Bonen A, Haynes FW, Graham TE. Substrate and hormonal responses to exercise in women using oral contraceptives. J Appl Physiol 1991, 70: 1917–27.
Finken MJ, Andrews RC, Andrew R, Walker BR. Cortisol metabolism in healthy young adults: sexual dimorphism in activities of A-ring reductases, but not 11 beta-hydroxysteroid dehydrogenases. J Clin Endocrinol Metab 1999, 84: 3316–21.
Atlaoui D, Duclos M, Gouarne C, Lacoste L, Barale F, Chatard JC. The 24-h urinary cortisol/cortisone ratio for monitoring training in elite swimmers. Med Sci Sports Exerc 2004, 36: 218–24.
Dovio A, Roveda E, Sciolla C, et al. Intense physical exercise increases systemic 11 beta-hydroxysteroid dehydrogenase type 1 activity in healthy adult subjects. Eur J Appl Physiol 2010, 108: 681–7.
Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev 2000, 21: 55–89.
Altemus M, Roca C, Galliven E, Romanos C, Deuster P. Increased vasopressin and adrenocorticotropin responses to stress in the midluteal phase of the menstrual cycle. J Clin Endocrinol Metab 2001, 86: 2525–30.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Boisseau, N., Enea, C., Diaz, V. et al. Oral contraception but not menstrual cycle phase is associated with increased free cortisol levels and low hypothalamo-pituitary-adrenal axis reactivity. J Endocrinol Invest 36, 955–964 (2013). https://doi.org/10.3275/8971
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3275/8971