Abstract
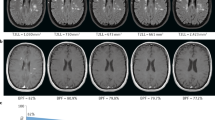
Magnetic resonance imaging (MRI) reveals that brain and spinal cord atrophy occur early in the course of multiple sclerosis (MS), far earlier than originally anticipated. This has important implications for the early treatment of patients with MS, as atrophy is thought to reflect destructive, irreversible pathology, and subclinical impairment if not overt disability. Several recent trials in MS have included atrophy as a secondary or exploratory measure of treatment efficacy. While measured cerebral volume or spinal cord area changes are small over 1 to 3 year intervals, they are sufficiently large that with current methodologies the atrophy measures should provide conclusive information as to the effectiveness of therapeutic interventions in halting progressive atrophy. Atrophy measures may also provide an important metric for the evaluation of disease in primary progressive MS, and in testing combined therapies and neuroprotective agents, where conventional MRI methodologies may be relatively weak.





Similar content being viewed by others
References
Whitaker JN, McFarland HF, Rudge P, et al. Outcomes assessment in multiple sclerosis clinical trials: a critical analysis. Mult Scler 1995; 1: 37–47
Miller DH, Albert PS, Barkhof F, et al. Guidelines for the use of magnetic resonance techniques in monitoring the treatment of multiple sclerosis. Ann Neurol 1996; 39: 6–16
Simon JH. Brain and spinal cord atrophy in multiple sclerosis. In: Frank JA, editor. Advances in multiple sclerosis. Neuroimaging Clin N Am 2000 Nov; 10: 753–69
Barnard RO, Triggs M. Corpus callosum in multiple sclerosis. J Neurol Neurosurg Psychiatry 1974; 37: 1259–64
Lumsden CE. The neuropathology of multiple sclerosis. In: Vinken PJ, Bruyn GW, editors. Multiple sclerosis and other demyelinating diseases. Handbook of clinical neurology. Vol. 9. Amsterdam: Elsevier, 1970: 217–309
Adams RD, Kubik CS. The morbid anatomy of the demyelinative diseases. Am J Med 1952; 12: 510–46
Simon JH, Jacobs LD, Campion A, et al. A longitudinal study of brain atrophy in relapsing multiple sclerosis. Neurology 1999; 53: 139–48
Brex PA, Jenkins R, Fox NC, et al. Detection of ventricular enlargement in patients at the earliest clinical stage of MS. Neurology 2000 Apr 25; 54(8): 1689–91
Rudick RA, Fisher E, Lee JC, et al. Use of the brain parenchymal fraction to measure whole brain atrophy in relapsingremitting MS. Multiple Sclerosis Collaborative Research Group. Neurology 1999; 53(8): 1698–704
Prineas JW. The neuropathology of multiple sclerosis. In: Koetsier JC, editor. Demyelinating diseases. Handbook of clinical neurology. Vol. 3. Amsterdam: Elsevier Science Publishers, 1985: 213–57
Prineas JW, Connell F, Raine CS. The fine structure of chronically active multiple sclerosis plaques. Neurology 1978; 28: 68–75
McDonald WI. Rachelle Fishman-Matthew Moore Lecture. The pathological and clinical dynamics of multiple sclerosis. J Neuropathol Exp Neurol 1994; Jul 53(4): 338–43
Ferguson B, Matyszak MK, Esiri MM, et al. Axonal damage in acute multiple sclerosis lesions. Brain 1997; 120: 393–9
Trapp BD, Peterson J, Ransohoff RM, et al. Axonal transection in the lesions of multiple sclerosis. N Engl J Med 1998; 338: 278–85
Arnold DL, Wolinsky JS, Matthews PM, et al. The use of magnetic resonance spectroscopy in the evaluation of the natural history of multiple sclerosis. J Neurol Neurosurg Psychiatry 1998; 64: S94–101
van Walderveen MAA, Kamphorst W, Scheltens P, et al. Histopathologic correlate of hypointense lesions on T1-weighted spin-echo MRI in multiple sclerosis. Neurology 1998; 50: 1282–8
Simon JH, Kinkel RP, Jacobs L, et al. A Wallerian degeneration pattern in patients at risk for MS. Neurology 2000; 54: 1155–60
Simon JH, Coombs B, Kinkel RP, et al. Tract degeneration patterns in MS. 52nd Annual Meeting of the American Academy of Neurology; 2000 Apr 29–May 6; San Diego (CA)
Simon JH. From enhancing lesions to brain atrophy in relapsing MS. J Neuroimmunol 1999; 98(1): 7–15
De Stefano N, Narayanan S, Matthews PM, et al. In vivo evidence for axonal dysfunction remote from focal cerebral demyelination of the type seen in multiple sclerosis. Brain 1999; 122 (Pt 10): 1933–9
Lee MA, Blamire AM, Pendlebury S, et al. Axonal injury or loss in the internal capsule and motor impairment in multiple sclerosis. Arch Neurol 2000; 57(1): 65–70
Brownell B, Hughes J T. The distribution of plaques in the cerebrum in multiple sclerosis. J Neurol Neurosurg Psychiatry 1962; 25: 315–20
Evangelou N, Esiri MM, Smith S, et al. Quantitative pathological evidence for axonal loss in normal appearing white matter in multiple sclerosis. Ann Neurol 2000; 47: 391–5
McGavern DB, Murray PD, Rivera-Quinones C, et al. Axonal loss results in spinal cord atrophy, electrophysiological abnormalities and neurological deficits following demyelination in a chronic inflammatory model of multiple sclerosis. Brain 2000; 123 (Pt 3): 519–31
Ge Y, Grossman RI, Udupa JK, et al. Glatiramer acetate (Copaxone) treatment in relapsing-remitting MS: quantitative MR assessment. Neurology 2000 Feb 22; 54(4): 813–7
Losseff NA, Wang L, Lai HM, et al. Progressive cerebral atrophy in multiple sclerosis: a serial MRI study. Brain 1996; 119 (Pt 6): 2009–19
Fox NC, Jenkins R, Leary S, et al. Progressive cerebral atrophy in MS: a serial study using registered, volumetric MRI. Neurology 2000 Feb 22; 54(4): 807–12
Stevenson VL, Leary SM, Losseff NA, et al. Spinal cord atrophy and disability in MS: a longitudinal study. Neurology 1998; 51(1): 234–8
Stevenson VL, Miller DH, Leary SM, et al. One year follow up study of primary and transitional progressive multiple sclerosis. J Neurol Neurosurg Psychiatry 2000; 68(6): 713–8
Davie CA, Barker GJ, Webb S, et al. Persistent functional deficit in multiple sclerosis and autosomal dominant cerebellar ataxia is associated with axon loss. Brain 1995; 118 (Pt 6): 1583–92
Filippi M, Campi A, Colombo B, et al. A spinal cord MRI study of benign and secondary progressive multiple sclerosis. J Neurol 1996; 243(7): 502–5
Filippi M, Mastronardo G, Rocca MA, et al. Quantitative volumetric analysis of brain magnetic resonance imaging from patients with multiple sclerosis. J Neurol Sci 1998; 158(2): 148–53
Liu C, Edwards S, Gong Q, et al. Three dimensional MRI estimates of brain and spinal cord atrophy in multiple sclerosis. J Neurol Neurosurg Psychiatry 1999; 66(3): 323–30
Paolillo A, Pozzilli C, Gasperini C, et al. Brain atrophy in relapsing-remitting multiple sclerosis: relationship with ‘black holes’, disease duration and clinical disability. J Neurol Sci 2000; 174(2): 85–91
Bakshi R, Czarnecki D, Shaikh ZA, et al. Brain MRI lesions and atrophy are related to depression in multiple sclerosis. Neuroreport 2000; 11: 1153–8
Phillips MD, Grossman RI, Miki Y, et al. Comparison of T2 lesion volume and magnetization transfer ratio histogram analysis and of atrophy and measures of lesion burden in patients with multiple sclerosis. Am J Neuroradiol 1998; 19(6): 1055–60
Kidd D, Thorpe JW, Thompson AJ, et al. Spinal cord MRI using multi-array coils and fast spin echo. II. Findings in multiple sclerosis. Neurology 1993; 43(12): 2632–7
Stevenson VL, Miller DH, Rovaris M. Primary and transitional progressive MS: a clinical and MRI cross-sectional study. Neurology 1999; 52(4): 839–45
Filippi M, Colombo B, Rovaris M, et al. A longitudinal magnetic resonance imaging study of the cervical cord in multiple sclerosis. J Neuroimaging 1997; 7(2): 78–80
IFNB Multiple Sclerosis Study Group and the University of British Columbia MS/MRI Analysis Group. Interferon beta-1b in the treatment of multiple sclerosis: final outcome of the randomized controlled trial. Neurology 1995; 45: 1277–85
Simon JH, Jacobs LD, Campion MK, et al. Magnetic resonance studies of intramuscular interferon beta-1a for relapsing multiple sclerosis. The Multiple Sclerosis Collaborative Research Group. Ann Neurol 1998; 43: 79–87
Filippi M, Paty DW, Kappos L, et al. Correlations between changes in disability and T2-weighted brain MRI activity in multiple sclerosis: a follow-up study. Neurology 1995; 45: 255–60
Truyen L, van Waesberghe JHTM, van Walderveen MAA, et al. Accumulation of hypointense lesions (‘black holes’) on T1 spin echo MRI correlates with disease progression in multiple sclerosis. Neurology 1996; 47: 1469–76
Simon JH, Lull J, Jacobs LD, et al. A longitudinal study of T1-hypointense lesions in relapsing MS: MSCRG trial of interferon beta-1a. Multiple Sclerosis Collaborative Research Group. Neurology 2000 Jul 25; 55(2): 185–92.
Edwards, SG, Gong QY, Liu C, et al. Infratentorial atrophy on magnetic resonance imaging and disability in multiple sclerosis. Brain 1999; 122 (Pt 2): 291–301
Losseff NA, Webb SL, O’Riordan J, et al. Spinal cord atrophy and disability in multiple sclerosis. A new reproducible and sensitive MRI method with potential to monitor disease progression. Brain 1996; 119 (Pt 3): 701–8
Lycklama A, Nijeholt GJ, Castelijns JA, et al. Magnetization transfer ratio of the spinal cord in multiple sclerosis: relationship to atrophy and neurologic disability. J Neuroimaging 2000; 10(2): 67–72
Hohol M, Guttmann CR, Orav J, et al. Serial neuropsychological assessment and magnetic resonance imaging analysis in multiple sclerosis. Arch Neurol 1997; 54: 1018–25
Rao SM. Neuropsychology of multiple sclerosis. Curr Opin Neurol 1995; 8: 216–20
Rao SM, Glatt S, Hammeke TA, et al. Chronic progressive multiple sclerosis: relationship between cerebral ventricular size and neuropsychological impairment. Arch Neurol 1985; 42: 678–82
Miller DE, Simon JH, Rudick RA, et al. Determinants of brain atrophy in relapsing multiple sclerosis [abstract]. Neurology 1999; 52: A3576
Paolillo A, Coles AJ, Molyneux PD, et al. Quantitative MRI in patients with secondary progressive MS treated with monoclonal antibody Campath 1H. Neurology 1999 11; 53(4): 751–7
Coles AJ, Wing MG, Molyneux P, et al. Monoclonal antibody treatment exposes three mechanisms underlying the clinical course of multiple sclerosis. Ann Neurol 1999; 46(3): 296–304
Thompson AJ, Polman CH, Miller DH, et al. Primary progressive multiple sclerosis. Brain 1997; 120: 1085–96
Simon JH. MR Imaging in the diagnosis of multiple sclerosis, elucidation of disease course and determining prognosis. In: Burks JS, Johnson KP, editors. Multiple sclerosis: diagnosis, medical management and rehabilitation. New York (NY): Demos Publishers, 2000: 99–126
Wolinsky JS, Narayana PA, Noseworthy JH, et al. Linomide in relapsing and secondary progressive MS: part II: MRI results. MRI Analysis Center of the University of Texas-Houston, Health Science Center, and the North American Linomide Investigators. Neurology 2000; 54: 1734–41
Gonen O, Catalaa I, Babb JS, et al. Total brain N-acetylaspartate: a new measure of disease load in MS. Neurology 2000 Jan 11; 54(1): 15–9
Richert ND, Frank JA. Magnetization transfer imaging to monitor clinical trials in multiple sclerosis. Neurology 1999; 53 (5 Suppl. 3): S29–32
Sormani MP, Iannucci G, Rocca MA, et al. Reproducibility of magnetization transfer ratio histogram-derived measures of the brain in healthy volunteers. Am J Neuroradiol 2000 Jan; 21(1): 133–6
Werring DJ, Clark CA, Barker GJ, et al. Diffusion tensor imaging of lesions and normal-appearing white matter in multiple sclerosis. Neurology 1999 May 12; 52(8): 1626–32
Simon, JH. The use of magnetic resonance imaging to select patients for multiple sclerosis trials. In: Filippi M, Grossman RI, Comi G, editors. Magnetic resonance techniques in clinical trials in multiple sclerosis. Milano: Springer-Verlag Italia, 1999: 21–36
Jacobs LD, Beck RW, Simon JH, et al. Intramuscular interferon beta-1a therapy initiated during a first demyelinating event in multiple sclerosis: CHAMPS Study Group. N Engl J Med 2000 Sep 28; 343(13): 898–904
Acknowledgements
Dr Simon has received honoraria as a consultant, and for talks, for Biogen Incorporated, Cambridge, MA, USA.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Simon, J.H. Brain and Spinal Cord Atrophy in Multiple Sclerosis. Mol Diag Ther 15, 427–436 (2001). https://doi.org/10.2165/00023210-200115060-00001
Published:
Issue Date:
DOI: https://doi.org/10.2165/00023210-200115060-00001