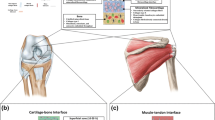
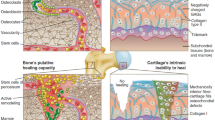
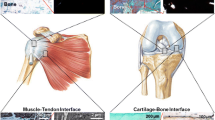
Abstract
Soft tissue-to-bone interfaces are complex structures that consist of gradients of extracellular matrix materials, cell phenotypes, and biochemical signals. These interfaces, called entheses for ligaments, tendons, and the meniscus, are crucial to joint function, transferring mechanical loads and stabilizing orthopedic joints. When injuries occur to connected soft tissue, the enthesis must be re-established to restore function, but due to structural complexity, repair has proven challenging. Tissue engineering offers a promising solution for regenerating these tissues. This prospective review discusses methodologies for tissue engineering the enthesis, outlined in three key design inputs: materials processing methods, cellular contributions, and biochemical factors.








Similar content being viewed by others
References
A.C. Abraham and T.L. Haut Donahue: From meniscus to bone: a quantitative evaluation of structure and function of the human meniscal attachments. Acta Biomater. 9, 6322–6329 (2013).
L. Mente and J.L. Lewis: Elastic modulus of calcified cartilage is an order of magnitude less than that of subchondral bone. J. Orthop. Res. 12, 637–647 (1994).
R.M. Schinagl, D. Gurskis, A.C. Chen, and R.L. Sah: Depth-dependent confined compression modulus of full-thickness bovine articular cartilage. J. Orthop. Res. 15, 499–506 (1997).
P. Brooks: Inflammation as an important feature of osteoarthritis. Bull. World Health Organ. 81, 689–690 (2003).
W.R. Shelton and A.D. Dukes: Meniscus replacement with bone anchors: a surgical technique. Arthrosc. J. Arthrosc. Relat. Surg. 10, 324–327 (1994).
E.A. Khetia and B.P. McKeon: Meniscal allografts: biomechanics and techniques. Sports Med. Arthrosc. 15, 114–120 (2007).
P.J. Yang and J.S. Temenoff: Engineering orthopedic tissue interfaces. Tissue Eng. B., Rev. 15, 127–141 (2009).
H.H. Lu and S. Thomopoulos: Functional attachment of soft tissues to bone: development, healing, and tissue engineering. Annu. Rev. Biomed. Eng. 15, 201–226 (2013).
S. Font Tellado, E. Rosado Balmayor, and M. Van Griensven: Strategies to engineer tendon/ligament-to-bone interface: biomaterials, cells and growth factors. Adv. Drug Deliv. Rev. 94, 126–140 (2015).
T.M. Hammoudi and J.S. Temenoff: Biomaterials for regeneration of tendons and ligaments. Biomater. Tissue Eng. Appl. 11, 307–341 (2011).
J. Gao, K. Messner, J. Ralphs, and M. Benjamin: An immunohistochemical study of enthesis development in the medial collateral ligament of the rat knee joint. Anat. Embryol. (Berlt). 19, 399–406 (1996).
J.P. Spalazzi, A.L. Boskey, N. Pleshko, and H.H. Lu: Quantitative mapping of matrix content and distribution across the ligament-to-bone insertion. PLoS ONE. 8, e74349 (2013).
S. Thomopoulos, G.M. Genin, and L.M. Galatz: The development and morphogenesis of the tendon-to-bone insertion. What development can teach us about healing. J. Musculoskelet. Neuronal Interact. 10, 35–45 (2010).
K. Messner and J. Gao: The menisci of the knee joint. Anatomical and functional characteristics, and a rationale for clinical treatment. J. Anat. 193, 161–178 (1998).
J. Gao: Immunolocalization of types I, II, and X collagen in the tibial insertion sites of the medial meniscus. Knee Surg. Sports Traumatol. Arthrosc. 8, 61–65 (2000).
W. Petersen and B. Tillmann: Structure and vascularization of the cruciate ligaments of the human knee joint. Anat. Embryol. (Berl). 200, 325–334 (1999).
I-N.E. Wang, S. Mitroo, F.H. Chen, H.H. Lu, and S.B. Doty: Age-dependent changes in matrix composition and organization at the ligament-to-bone insertion. J. Orthop. Res. 24, 1745–1755 (2006).
M. Benjamin and J.R. Ralphs: The cell and developmental biology of tendons and ligaments. Int. Rev. Cytol. 196, 85–130 (2000).
G.M. Genin, A. Kent, V. Birman, B. Wopenka, J.D. Pasteris, P.J. Marquez, and S. Thomopoulos: Functional grading of mineral and collagen in the attachment of tendon to bone. Biophys. J. 97, 976–985 (2009).
P. Buma, N.N. Ramrattan, T.G. van Tienen, and R.P. Veth: Tissue engineering of the meniscus. Biomaterials 25, 1523–1532 (2004).
M.T. Rodrigues, R.L. Reis, and M.E. Gomes: Engineering tendon and ligament tissues: present developments towards successful clinical products. J. Tissue Eng. Regen. Med. 7, 673–686 (2013).
A. Di Luca, C.A. Van Blitterswijk, and L. Moroni: The osteochondral interface as a gradient tissue: From development to the fabrication of gradient scaffolds for regenerative medicine. Birth Defects Res. C 105, 34–52 (2015).
C. Vaquette, W. Fan, Y. Xiao, S. Hamlet, D.W. Hutmacher, and S. Ivanovski: A biphasic scaffold design combined with cell sheet technology for simultaneous regeneration of alveolar bone/periodontal ligament complex. Biomaterials 33, 5560–5573 (2012).
A.D. Waggett, J.R. Ralphs, A.P.L. Kwan, D. Woodnutt, and M. Benjamin: Characterization of collagens and proteoglycans at the insertion of the human Achilles tendon. Matrix Biol. 16, 457–470 (1998).
P. Fratzl and R. Weinkamer: Nature’s hierarchical materials. Prog. Mater. Sci. 52, 1263–1334 (2007).
K.L. Moffat, W-H.S. Sun, P.E. Pena, N.O. Chahine, S.B. Doty, G.A. Ateshian, C.T. Hung, and H.H. Lu: Characterization of the structure-function relationship at the ligament-to-bone interface. Proc. Natl. Acad. Sci. U.S.A. 105, 7947–7952 (2008).
Y.X. Liu, S. Thomopoulos, V. Birman, J.S. Li, and G.M. Genin: Bi-material attachment through a compliant interfacial system at the tendon-to-bone insertion site. Mech. Mater. 44, 83–92 (2012).
G. Shen: The role of type X collagen in facilitating and regulating endochondral ossification of articular cartilage. Orthod. Craniofacial Res. 8, 11–17 (2005).
T.E. Hardingham and A.J. Fosang: Proteoglycans: many forms and many function. FASEB J. 6, 861–870 (1992).
M. Benjamin and J.R. Ralphs: Fibrocartilage in tendons and ligaments—an adaptation to compressive load. J. Anat. 193(Pt 4), 481–494 (1998).
J. Melrose, S. Smith, M. Cake, R. Read, and J. Whitelock: Comparative spatial and temporal localisation of perlecan, aggrecan and type I, II and IV collagen in the ovine meniscus: An ageing study. Histochem. Cell Biol. 124, 225–235 (2005).
L. Rossetti, L.A. Kuntz, E. Kunold, J. Schock, H. Grabmayr, S.A. Sieber, R. Burgkart, and A.R. Bausch: The microstructure and micromechanics of the tendon–bone insertion. Nat. Mater. 16, 664–670 (2017).
H. Tavakoli Nia, L. Han, I. Soltani Bozchalooi, P. Roughley, K. Youcef-Toumi, A.J. Grodzinsky, and C. Ortiz: Aggrecan nanoscale solid-fluid interactions are a primary determinant of cartilage dynamic mechanical properties. ACS Nano. 9, 2614–2625 (2015).
A.K. Garg, R.A. Berg, F.H. Silver, and H.G. Garg: Effect of proteoglycans on type I collagen fibre formation. Biomaterials 10, 413–419 (1989).
K.G. Vogel and J.A. Trotter: The effect of proteoglycans on the morphology of collagen fibrils formed in vitro. Coll. Relat. Res. 7, 105–114 (1987).
K.G. Vogel, M. Paulsson, and D. Heinegård: Specific inhibition of type I and type II collagen fibrillogenesis by the small proteoglycan of tendon. Biochem. J. 223, 587–597 (1984).
E.J. Vanderploeg, C.G. Wilson, S.M. Imler, C.H.Y. Ling, and M.E. Levenston: Regional variations in the distribution and colocalization of extracellular matrix proteins in the juvenile bovine meniscus. J. Anat. 221, 174–186 (2012).
B. Wopenka and J.D. Pasteris: A mineralogical perspective on the apatite in bone. Mater. Sci. Eng. C 25, 131–143 (2005).
F. Nudelman, A.J. Lausch, N.A. Sommerdijk, and E.D. Sone: In vitro models of collagen biomineralization. J. Struct. Biol. 183, 258–269 (2013).
S. Weiner and H.D. Wagner: THE MATERIAL BONE: structure-mechanical function relations. Annu. Rev. Mater. Sci. 28, 271–298 (1998).
N. Reznikov, R. Shahar, and S. Weiner: Bone hierarchical structure in three dimensions. Acta Biomater. 10, 3815–3826 (2014).
A.G. Schwartz, J.D. Pasteris, G.M. Genin, T.L. Daulton, and S. Thomopoulos: Mineral distributions at the developing tendon enthesis. PLoS ONE 7, 1–11 (2012).
T.M. Keaveny, E.F. Morgan, G.L. Niebur, and O.C. Yeh: Biomechanics of trabecular bone. Annu. Rev. Biomed. Eng. 3, 307–333 (2001).
A.C. Deymier-Black, J.D. Pasteris, G.M. Genin, and S. Thomopoulos: Allometry of the tendon enthesis: mechanisms of load transfer between tendon and bone. J. Biomech. Eng. 137, 111005 (2015).
K.S. Allan, R.M. Pilliar, J. Wang, M.D. Grynpas, and R.A. Kandel: Formation of biphasic constructs containing cartilage with a calcified zone interface. Tissue Eng. 13, 167–177 (2007).
X. Huang, D. Yang, W. Yan, Z. Shi, J. Feng, Y. Gao, W. Weng, and S. Yan: Osteochondral repair using the combination of fibroblast growth factor and amorphous calcium phosphate/poly(l-lactic acid) hybrid materials. Biomaterials 28, 3091–3100 (2007).
W. Liu, J. Lipner, J. Xie, C.N. Manning, S. Thomopoulos, and Y. Xia: Nano fiber scaffolds with gradients in mineral content for spatial control of osteogenesis. ACS Appl. Mater. Interfaces 6, 2842–2849 (2014).
B.S. Kim, E.J. Kim, J.S. Choi, J.H. Jeong, C.H. Jo, and Y.W. Cho: Human collagen-based multilayer scaffolds for tendon-to-bone interface tissue engineering. J. Biomed. Mater. Res. A 102, 4044–4054 (2014).
E. Nyberg, A. Rindone, A. Dorafshar, and W.L. Grayson: Comparison of 3D-printed poly-ε-caprolactone scaffolds functionalized with tricalcium phosphate, hydroxyapatite, bio-oss, or decellularized bone matrix. Tissue Eng. A 23, 503–514 (2017).
A. Tevlek, P. Hosseinian, C. Ogutcu, M. Turk, and H.M. Aydin: Bi-layered constructs of poly(glycerol-sebacate)-β-tricalcium phosphate for bone-soft tissue interface applications. Mater. Sci. Eng. C 72, 316–324 (2017).
J.P. Spalazzi, S.B. Doty, K.L. Moffat, W.N. Levine, and H.H. Lu: Development of controlled matrix heterogeneity on a triphasic scaffold for orthopedic interface tissue engineering. Tissue Eng. 12, 3497–3508 (2006).
G. Criscenti, A. Longoni, A. Di Luca, C. De Maria, C.A. Van Blitterswijk, G. Vozzi, and L. Moroni: Triphasic scaffolds for the regeneration of the bone–ligament interface. Biofabrication 8, 15009 (2016).
J.A. Cooper, H.H. Lu, F.K. Ko, J.W. Freeman, and C.T. Laurencin: Fiber-based tissue-engineered scaffold for ligament replacement: design considerations and in vitro evaluation. Biomaterials 26, 1523–1532 (2005).
N.H. Dormer, M. Singh, L. Zhao, N. Mohan, C.J. Berkland, and M.S. Detamore: Osteochondral interface regeneration of the rabbit knee with macroscopic gradients of bioactive signals. J. Biomed. Mater. Res. 100, 162–170 (2012).
Y. Liu, S. Thomopoulos, C. Chen, V. Birman, M.J. Buehler, and G.M. Genin: Modelling the mechanics of partially mineralized collagen fibrils, fibres and tissue. J. R. Soc. Interface 11, 20130835 (2014).
J.Z. Paxton, K. Donnelly, R.P. Keatch, and K. Baar: Engineering the bone–ligament Interface using polyethylene glycol diacrylate incorporated with hydroxyapatite. Tissue Eng. A 15, 1201–1209 (2009).
G.H. Altman, R.L. Horan, H.H. Lu, J. Moreau, I. Martin, J.C. Richmond, and D.L. Kaplan: Silk matrix for tissue engineered anterior cruciate ligaments. Biomaterials 23, 4131–4141 (2002).
S. Font Tellado, W. Bonani, E. Rosado Balmayor, P. Föhr, A. Motta, C. Migliaresi, and M. van Griensven: Fabrication and characterization of biphasic silk fibroin scaffolds for tendon/ligament-to-bone tissue engineering. Tissue Eng. A 23, 859–872 (2017).
Y-B. Park, C-W. Ha, C-H. Lee, and Y-G. Park: Restoration of a large osteochondral defect of the knee using a composite of umbilical cord blood-derived mesenchymal stem cells and hyaluronic acid hydrogel: a case report with a 5-year follow-up. BMC Musculoskelet. Disord. 18, 59 (2017).
M.C. McCorry, M.M. Mansfield, X. Sha, D.J. Coppola, J.W. Lee, and L.J. Bonassar: A model system for developing a tissue engineered meniscal enthesis. Acta Biomater. 56, 110–117 (2016).
C.H. Chang, F.H. Lin, C.C. Lin, C.H. Chou, and H.C. Liu: Cartilage tissue engineering on the surface of a novel gelatin-calcium- phosphate biphasic scaffold in a double-chamber bioreactor. J. Biomed. Mater. Res.B, Appl. Biomater. 71, 313–321 (2004).
I-N.E. Wang, J. Shan, R. Choi, S. Oh, C.K. Kepler, F.H. Chen, and H.H. Lu: Role of osteoblast–fibroblast interactions in the formation of the ligament-to-bone interface. J. Orthop. Res. 25, 1609–1620 (2007).
K. Xu, L.A. Kuntz, P. Foehr, K. Kuempel, A. Wagner, J. Tuebel, C.V. Deimling, and R.H. Burgkart: Efficient decellularization for tissue engineering of the tendon-bone interface with preservation of biomechanics. PLoS ONE. 12, e0171577 (2017).
S. Sundar, C.J. Pendegrass, and G.W. Blunn: Tendon bone healing can be enhanced by demineralized bone matrix: a functional and histological study. J. Biomed. Mater. Res. B, Appl. Biomater. 88, 115–122 (2009).
J. Lipner, H. Shen, L. Cavinatto, W. Liu, N. Havlioglu, Y. Xia, L.M. Galatz, and S. Thomopoulos: In vivo evaluation of adipose-derived stromal cells delivered with a nanofiber scaffold for tendon-to-bone repair. Tissue Eng. A 21, 2766–2774 (2015).
U.G. Wegst, H. Bai, E. Saiz, A.P. Tomsia, and R.O. Ritchie: Bioinspired structural materials. Nat. Mater. 14, 23–36 (2014).
X. Ding, M. Zhu, B. Xu, J. Zhang, Y. Zhao, S. Ji, L. Wang, L. Wang, X. Li, D. Kong, X. Ma, and Q. Yang: Integrated trilayered silk fibroin scaffold for osteochondral differentiation of adipose-derived stem cells. ACS Appl. Mater. Interfaces 6, 16696–16705 (2014).
J. Ma, M.J. Smietana, T.Y. Kostrominova, E.M. Wojtys, L.M. Larkin, and E.M. Arruda: Three-dimensional engineered bone–ligament–bone constructs for anterior cruciate ligament replacement. Tissue Eng. A 18, 103–116 (2012).
H.K. Min, S.H. Oh, J.M. Lee, G.I. Im, and J.H. Lee: Porous membrane with reverse gradients of PDGF-BB and BMP-2 for tendon-to-bone repair: In vitro evaluation on adipose-derived stem cell differentiation. Acta Biomater. 10, 1272–1279 (2014).
B.M. Baker and R.L. Mauck: The effect of nanofiber alignment on the maturation of engineered meniscus constructs. Biomaterials 28, 1967–1977 (2007).
J.S. Park, H.J. Yang, D.G. Woo, H.N. Yang, K. Na, and K.H. Park: Chondrogenic differentiation of mesenchymal stem cells embedded in a scaffold by long-term release of TGF-B3 complexed with chondroitin sulfate. J. Biomed. Mater. Res. A 92, 806–816 (2010).
W.M. Han, S-J. Heo, T.P. Driscoll, J.F. Delucca, C.M. McLeod, L.J. Smith, R.L. Duncan, R.L. Mauck, and D.M. Elliott: Microstructural heterogeneity directs micromechanics and mechanobiology in native and engineered fibrocartilage. Nat. Mater. 15, 477–484 (2016).
J.P. Spalazzi, E. Dagher, S.B. Doty, X.E. Guo, S.A. Rodeo, and H.H. Lu: In vivo evaluation of a multiphased scaffold designed for orthopaedic interface tissue engineering and soft tissue-to-bone integration. J. Biomed. Mater. Res. A 86, 1–12 (2008).
J.L. Puetzer, E. Koo, and L.J. Bonassar: Induction of fiber alignment and mechanical anisotropy in tissue engineered menisci with mechanical anchoring. J. Biomech. 48, 1436–1443 (2015).
J.B. Lian and G.S. Stein: Concepts of osteoblast growth and differentiation: basis for modulation of bone cell development and tissue formation. Crit. Rev. Oral Biol. Med. 3, 269–305 (1992).
E.J. Mackie, Y.A. Ahmed, L. Tatarczuch, K.S. Chen, and M. Mirams: Endochondral ossification: How cartilage is converted into bone in the developing skeleton. Int. J. Biochem. Cell Biol. 40, 46–62 (2008).
L.J. Sandell and T. Aigner: Articular cartilage and changes in arthritis. An introduction: Cell biology of osteoarthritis. Arthritis Res. 3, 107–113 (2001).
J. Sanchez-Adams and K.A. Athanasiou: The knee meniscus: a complex tissue of diverse cells. Cell. Mol. Bioeng. 2, 332–340 (2009).
K. Spanoudes, D. Gaspar, A. Pandit, and D.I. Zeugolis: The biophysical, biochemical, and biological toolbox for tenogenic phenotype maintenance in vitro. Trends Biotechnol. 32, 474–482 (2014).
A. Hasegawa, H. Nakahara, M. Kinoshita, H. Asahara, J. Koziol, and M.K. Lotz: Cellular and extracellular matrix changes in anterior cruciate ligaments during human knee aging and osteoarthritis. Arthritis Res. Ther. 15, R29 (2013).
C.H. Lee, S.A. Rodeo, L.A. Fortier, C. Lu, C. Erisken, and J.J. Mao: Protein-releasing polymeric scaffolds induce fibrochondrocytic differentiation of endogenous cells for knee meniscus regeneration in sheep. Sci. Transl. Med. 6, 266ra171 (1–11) (2014).
F.A. Monibi and J.L. Cook: Tissue-derived extracellular matrix bioscaffolds: emerging applications in cartilage and meniscus repair. Tissue Eng. B, Rev. 23, 386–398 (2017).
E. Zelzer, E. Blitz, M.L. Killian, and S. Thomopoulos: Tendon-to-bone attachment: from development to maturity. Birth Defects Res. Part C. 102, 101–112 (2014).
J. Jiang, S.B. Nicoll, and H.H. Lu: Co-culture of osteoblasts and chondrocytes modulates cellular differentiation in vitro. Biochem. Biophys. Res. Commun. 338, 762–770 (2005).
M.C. McCorry, J.L. Puetzer, and L.J. Bonassar: Characterization of mesenchymal stem cells and fibrochondrocytes in three-dimensional co-culture: analysis of cell shape, matrix production, and mechanical performance. Stem Cell Res. Ther. 7, 39 (2016).
M.C. McCorry and L.J. Bonassar: Fiber development and matrix production in tissue-engineered menisci using bovine mesenchymal stem cells and fibrochondrocytes. Connect. Tissue Res. 58, 329–341 (2017).
G. Im: Coculture in musculoskeletal tissue regeneration. Tissue Eng. B, Rev. 20, 545–554 (2014).
L. Bian, D.Y. Zhai, R.L. Mauck, and J.A. Burdick: Coculture of human mesenchymal stem cells and enhances functional properties of engineered cartilage reverse primer. Tissue Eng. A 17, 1137–1145 (2011).
G.M. Hoben, V.P. Willard, and K.A. Athanasiou: Fibrochondrogenesis of hESCs: growth factor combinations and cocultures. Stem Cells Dev. 18, 283–292 (2009).
E. Gardner and R. O’Rahilly: The early development of the knee joint in staged human embryos. J. Anat. 102, 289–299 (1968).
D.J. Gray and E. Gardner: Prenatal development of the human knee and superior tibiofibular joints. Am. J. Anat. 86, 235–287 (1950).
J.A. Mérida-Velasco, I. Sánchez-Montesinos, J. Espín-Ferra, J.F. Rodríguez-Vázquez, J.R. Mérida-Velasco, and J. Jiménez-Collado: Development of the human knee joint. Anat. Rec. 248, 269–278 (1997).
A.I. Caplan and J.E. Dennis: Mesenchymal stem cells as trophic mediators. J. Cell. Biochem. 98, 1076–1084 (2006).
A.M. Mackay, S.C. Beck, J.M. Murphy, F.P. Barry, C.O. Chichester, and M.F. Pittenger: Chondrogenic differentiation of cultured human mesenchymal stem cells from marrow. Tissue Eng. 4, 415–428 (1998).
G.I. Im, Y.W. Shin, and K.B. Lee: Do adipose tissue-derived mesenchymal stem cells have the same osteogenic and chondrogenic potential as bone marrow-derived cells? Osteoarthr. Cartil. 13, 845–853 (2005).
J.P. Spalazzi and H.H. Lu: Osteoblast and chondrocyte interactions during coculture on scaffolds. IEEE Eng. Med. Biol. Mag. 22, 27–34 (2003).
N.J. Gunja and K.A. Athanasiou: Passage and reversal effects on gene expression of bovine meniscal fibrochondrocytes. Arthritis Res. Ther. 9, 1–12 (2007).
C. Zeltz and D. Gullberg: The integrin-collagen connection—a glue for tissue repair? J. Cell Sci. 129, 653–664 (2016).
A.D. Augst, H.J. Kong, and D.J. Mooney: Alginate hydrogels as biomaterials. Macromol. Biosci. 6, 623–633 (2006).
B.M. Baker, A.S. Nathan, A.O. Gee, R.L. Mauck: The influence of an aligned nanofibrous topography on human mesenchymal stem cell fibrochondrogenesis. Biomaterials 31, 6190–6200 (2010).
J.R. Tse, and A.J. Engler: Stiffness gradients mimicking in vivo tissue variation regulate mesenchymal stem cell fate. PLoS ONE 6, e15978 (2011).
A.J. Engler, S. Sen, H.L. Sweeney, and D.E. Discher: Matrix elasticity directs stem cell lineage specification. Cell 126, 677–689 (2006).
C.H. Lee, H.J. Shin, I.H. Cho, Y-M. Kang, I.A. Kim, K-D. Park, and J-W. Shin: Nanofiber alignment and direction of mechanical strain affect the ECM production of human ACL fibroblast. Biomaterials 26, 1261–1270 (2005).
S.D. Subramony, B.R. Dargis, M. Castillo, E.U. Azeloglu, M.S. Tracey, A. Su, and H.H. Lu: The guidance of stem cell differentiation by substrate alignment and mechanical stimulation. Biomaterials 34, 1942–1953 (2013).
J.E. Phillips, K.L. Burns, J.M. Le Doux, R.E. Guldberg, and A.J. García: Engineering graded tissue interfaces. Proc. Natl. Acad. Sci. USA 105, 12170–12175 (2008).
M. Urist, R. DeLange, and G. Finerman: Bone cell differentiation and growth factors. Science 220, 680–686 (1983).
T.A. Linkhart, S. Mohan, and D.J. Baylink: Growth factors for bone growth and repair: IGF, TGF and BMP. Bone 19, 1S–12S (1996).
J.R. Lieberman, A. Daluiski, and T.A. Einhorn: The Role of growth factors in the repair of bone. J. Bone oin Surg. 84, 1032–1044 (2002).
T. Molloy, Y. Wang, and G.A.C. Murrell: The roles of growth factors in tendon and ligament healing. Sport. Med. 33, 381–394 (2003).
R. James, G. Kesturu, G. Balian, and A.B. Chhabra: Tendon: biology, biomechanics, repair, growth factors, and evolving treatment options. J. Hand Surg. Am. 33, 102–112 (2008).
P.M. Van der Kraan, P. Buma, T. Van Kuppevelt, and W.B. Van Den Berg: Interaction of chondrocytes, extracellular matrix and growth factors: relevance for articular cartilage tissue engineering. Osteoarthr. Cartil. 10, 631–637 (2002).
D.J. Baylink, R.D. Finkelman, and S. Mohan: Growth factors to stimulate bone formation. J. Bone Miner. Res. 8, S565–S572 (1993).
C. Li, C. Vepari, H-J. Jin, H.J. Kim, and D.L. Kaplan: Electrospun silk-BMP-2 scaffolds for bone tissue engineering. Biomaterials 27, 3115–3124 (2006).
N. Kakudo, K. Kusumoto, Y.B. Wang, Y. Iguchi, and Y. Ogawa: Immunolocalization of vascular endothelial growth factor on intramuscular ectopic osteoinduction by bone morphogenetic protein-2. Life Sci. 79, 1847–1855 (2006).
Z.S. Patel, S. Young, Y. Tabata, J.A. Jansen, M.E.K. Wong, and A.G. Mikos: Dual delivery of an angiogenic and an osteogenic growth factor for bone regeneration in a critical size defect model. Bone 43, 931–940 (2008).
J.A. Parry, M.G.L. Olthof, K.L. Shogren, M. Dadsetan, A. Van Wijnen, M. Yaszemski, and S. Kakar: Three-dimension-printed porous poly(propylene fumarate) scaffolds with delayed rhBMP-2 release for anterior cruciate ligament graft fixation. Tissue Eng. A 0, 1–7 (2017).
N. Indrawattana, G. Chen, M. Tadokoro, L.H. Shann, H. Ohgushi, T. Tateishi, J. Tanaka, and A. Bunyaratvej: Growth factor combination for chondrogenic induction from human mesenchymal stem cell. Biochem. Biophys. Res. Commun. 320, 914–919 (2004).
P. Yilgor, K. Tuzlakoglu, R.L. Reis, N. Hasirci, and V. Hasirci: Incorporation of a sequential BMP-2/BMP-7 delivery system into chitosan-based scaffolds for bone tissue engineering. Biomaterials 30, 3551–3559 (2009).
J.T. Connelly, C.G. Wilson, and M.E. Levenston: Characterization of proteoglycan production and processing by chondrocytes and BMSCs in tissue engineered constructs. Osteoarthr. Cartil. 16, 1092–1100 (2008).
B. Johnstone, T.M. Hering, A.I. Caplan, V.M. Goldberg, and J.U. Yoo: In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp. Cell Res. 238, 265–272 (1998).
R.F. Macbarb, E.A. Makris, J.C. Hu, and K.A. Athanasiou: A chondroitinase-ABC and TGF-β1 treatment regimen for enhancing the mechanical properties of tissue-engineered fibrocartilage. Acta Biomater. 9, 4626–4634 (2012)
R.P. Marini, I. Martin, M.M. Stevens, R. Langer, and V.P. Shastri: FGF-2 enhances TGF-B1 induced periosteal chondrogenesis. J. Orthop. Res. 22, 1114–1119 (2004).
S.M. Imler, A.N. Doshi, and M.E. Levenston: Combined effects of growth factors and static mechanical compression on meniscus explant biosynthesis. Osteoarthr. Cartil. 12, 736–744 (2004).
A. Augst, D. Marolt, L.E. Freed, C. Vepari, L. Meinel, M. Farley, R. Fajardo, N. Patel, M. Gray, D.L. Kaplan, and G. Vunjak-Novakovic: Effects of chondrogenic and osteogenic regulatory factors on composite constructs grown using human mesenchymal stem cells, silk scaffolds and bioreactors. J. R. Soc. Interface 5, 929–939 (2008).
H. Park, J.S. Temenoff, T.A. Holland, Y. Tabata, and A.G. Mikos: Delivery of TGF-1 and chondrocytes via injectable, biodegradable hydrogels for cartilage tissue engineering applications. Biomaterials 26, 7095–7103 (2005).
M.B. Mueller, M. Fischer, J. Zellner, A. Berner, T. Dienstknecht, L. Prantl, R. Kujat, M. Nerlich, R.S. Tuan, and P. Angele: Hypertrophy in mesenchymal stem cell chondrogenesis: effect of TGF-β isoforms and chondrogenic conditioning. Cells Tissues Organs 192, 158–166 (2010).
M. Kim, I.E. Erickson, M. Choudhury, N. Pleshko, and R.L. Mauck: Transient exposure to TGF-B3 improves the functional chondrogenesis of MSC-laden hyaluronic acid hydrogels. J. Mech. Behav. Biomed. Mater. 11, 92–101 (2012).
E. Farng, A.R.U. Bs, D.B. Bs, S.E. Bs, and D.R. Mcallister: The effects of GDF-5 and uniaxial strain on mesenchymal stem cells in 3-D culture. Clin. Orthop. Relat. Res. 466, 1930–1937 (2008).
R. James, S.G. Kumbar, C.T. Laurencin, G. Balian, and A.B. Chhabra: Tendon tissue engineering: adipose-derived stem cell and GDF-5 mediated regeneration using electrospun matrix systems. Biomed. Mater. 6, 25011 (2011).
N.I. Aguilar, S. Trippel, S. Shi, and L.J. Bonassar: Customized biomaterials to augment chondrocyte gene therapy. Acta Biomater. 53, 260–267 (2017).
J.L. Puetzer, B.N. Brown, J.J. Ballyns, and L.J. Bonassar: The effect of IGF-I on anatomically shaped tissue-engineered menisci. Tissue Eng. A 19, 1443–1450 (2013).
S. Thomopoulos, F.L. Harwood, M.J. Silva, D. Amiel, and R.H. Gelberman: Effect of several growth factors on canine flexor tendon fibroblast proliferation and collagen synthesis in vitro. J. Hand Surg. Am. 30, 441–447 (2005).
C.K. Hee, J.S. Dines, L.A. Solchaga, V.R. Shah, and J.O. Hollinger: Regenerative tendon and ligament healing: opportunities with recombinant human platelet-derived growth factor BB-homodimer. Tissue Eng. B, Rev. 18, 225–234 (2012).
B.S. Yoon, R. Pogue, D.A. Ovchinnikov, I. Yoshii, Y. Mishina, R.R. Behringer, and K.M. Lyons: BMPs regulate multiple aspects of growth-plate chondrogenesis through opposing actions on FGF pathways. Development 133, 4667–4678 (2006).
E. Minina, C. Kreschel, M.C. Naski, D.M. Ornitz, and A. Vortkamp: Interaction of FGF, Ihh/Pthlh, and BMP signaling integrates chondrocyte proliferation and hypertrophic differentiation. Dev. Cell 3, 439–449 (2002).
P.C. Bessa, M. Casal, and R.L. Reis: Bone morphogenetic proteins in tissue engineering: the road from laboratory to clinic, part II (BMP delivery). J. Tissue Eng. Regen. Med. 2, 81–96 (2008).
N.M. Wolfman, G. Hattersley, K. Cox, A.J. Celeste, R. Nelson, N. Yamaji, J.L. Dube, E. Diblasio-smith, J. Nove, J.J. Song, J.M. Wozney, V. Rosen, N.M. Wolfman, G. Hattersley, K. Cox, and J. Anthony: Ectopic induction of tendon and ligament in rats by growth and differentiation factors 5, 6, and 7, members of the TGF-β gene family. J. Clin. Invest. 100, 321–330 (1997).
D. Chen, M. Zhao, G.R. Mundy, D. Chen, M. Zhao, G.R. Mundy, and B. Morphogenetic: Bone morphogenetic proteins. Growth Factors 22, 233–241 (2004).
R.S. Decker, H-B. Um, N.A. Dyment, N. Cottingham, Y. Usami, M. Enomoto-Iwamoto, M.S. Kronenberg, P. Maye, D.W. Rowe, E. Koyama, and M. Pacifici: Cell origin, volume and arrangement are drivers of articular cartilage formation, morphogenesis and response to injury in mouse limbs. Dev. Biol. 426, 56–68 (2017).
U. Heine, E.F. Munoz, K.C. Flanders, L.R. Ellingsworth, H.Y. Lam, N.L. Thompson, A.B. Roberts, and M.B. Sporn: Role of transforming growth factor-beta in the development of the mouse embryo. J. Cell Biol. 105, 2861–2876 (1987).
C.M. Leonard, H.M. Fuld, D.A. Frenz, S.A. Downie, J. Massague, and S.A. Newman: Role of transforming growth factor-B in chondrogenic pattern formation in the embryonic limb: stimulation of mesenchymal condensation and fibronectin gene expression by exogenous TGF-B and evidence for endogenous TGF-B-like activity. Dev. Biol. 145, 99–109 (1991).
W.M. Kulyk, B.J. Rodgers, K. Greer, and R.A. Kosher: Promotion of embryonic chick limb cartilage differentiation by transforming growth factor-B. Dev. Biol. 135, 424–430 (1989).
O.G.P. Isaksson, J-O. Jansson, and I.A.M. Gause: Growth hormone stimulates longitudinal bone growth. Science 216, 1237–1239 (1982).
S. Mohan, Y. Nakao, Y. Honda, E. Landale, U. Leser, C. Dony, K. Lang, and D.J. Baylink: Studies on the mechanisms by which insulin-like growth factor (IGF) binding protein-4 (IGFBP-4) and IGFBP-5 modulate IGF actions in bone cells. J. Biol. Chem. 270, 20424–20431 (1995).
E.B. Hunziker, J. Wagner, and J. Zapf: Differential effects of insulin-like growth factor I and growth hormone on developmental stages of rat growth plate chondrocytes in vivo. J. Clin. Invest. 93, 1078–1086 (1994).
S.O. Abrahamsson: Similar effects of recombinant human insulin-like growth factor-I and II on cellular activities in flexor tendons of young rabbits: Experimental studies in vitro. J. Orthop. Res. 15, 256–262 (1997).
V. Midy and J. Plouët: Vasculotropin/vascular endothelial growth factor induces differentiation in cultured osteoblasts. Biochem. Biophys. Res. Commun. 199, 380–386 (1994).
D.W. Leung, G. Cachianes, W.J. Kuang, D.V. Goeddel, and N. Ferrara: Vascular endothelial growth factor is a secreted angiogenic mitogen. Science 246, 1306–1309 (1989).
P.J. Keck, S.D. Hauser, G. Krivi, K. Sanzo, T. Warren, J. Feder, and D.T. Connolly: Vascular permeability factor, an endothelial cell mitogen related to PDGF. Science 246, 1309–1312 (1989).
S.K.C. Sundararaj, R.D. Cieply, G. Gupta, T.A. Milbrandt, and D.A. Puleo: Treatment of growth plate injury using IGF-1 loaded PLGA scaffold. J. Tissue Eng. Regen. Med. 9, E202–E209 (2015).
K.J. Gooch, T. Blunk, D.L. Courter, A.L. Sieminski, P.M. Bursac, G. Vunjak-Novakovic, and L.E. Freed: IGF-I and mechanical environment interact to modulate engineered cartilage development. Biochem. Biophys. Res. Commun. 286, 909–915 (2001).
W.L. Murphy, M.C. Peters, D.H. Kohn, and D.J. Mooney: Sustained release of vascular endothelial growth factor from mineralized poly(lactide-co-glycolide) scaffolds for tissue engineering. Biomaterials 21, 2521–2527 (2000).
A.F. Steinert, G.D. Palmer, R. Capito, J.G. Hofstaetter, C. Pilapil, S.C. Ghivizzani, M. Spector, and C.H. Evans: Genetically enhanced engineering of meniscus tissue using ex vivo delivery of transforming growth factor-beta 1 complementary deoxyribonucleic acid. Tissue Eng. 13, 2227–2237 (2007).
H.V. Almeida, Y. Liu, G.M. Cunniffe, K.J. Mulhall, A. Matsiko, C.T. Buckley, F.J. O’Brien, and D.J. Kelly: Controlled release of transforming growth factor-β3 from cartilage-extra-cellular-matrix-derived scaffolds to promote chondrogenesis of human-joint-tissue-derived stem cells. Acta Biomater. 10, 4400–4409 (2014).
A. Hildebrand, M. Romarís, L.M. Rasmussen, D. Heinegård, D.R. Twardzik, W.A. Border, and E. Ruoslahti: Interaction of the small interstitial proteoglycans biglycan, decorin and fibromodulin with transforming growth factor beta. Biochem. J. 302, 527–534 (1994).
W-N. Qi and S.P. Scully: Extracellular collagen regulates expression of trasforming growth factor-beta1 gene. J. Orthop. Res. 18, 928–932 (2000).
M. Mongiat, J. Otto, R. Oldershaw, F. Ferrer, J.D. Sato, and R.V. Iozzo: Fibroblast growth factor-binding protein is a novel partner for perlecan protein core. J. Biol. Chem. 276, 10263–10271 (2001).
R. Ruppert, E. Hoffmann, and W. Sebald: Human bone morphogenetic protein 2 contains a heparin-binding site which modifies its biological activity. Eur. J. Biochem. 237, 295–302 (1996).
Y. Zhu, A. Oganesian, D.R. Keene, and L.J. Sandell: Type IIA procollagen containing the cysteine-rich amino propeptide is deposited in the extracellular matrix of prechondrogenic tissue and binds to TGF-B1 and BMP-2. J. Cell Biol. 144, 1069–1080 (1999).
M. Kawecki, W. Łabuś, A. Klama-Baryla, D. Kitala, M. Kraut, J. Glik, M. Misiuga, M. Nowak, T. Bielecki, and A. Kasperczyk: A review of decellurization methods caused by an urgent need for quality control of cell-free extracellular matrix’ scaffolds and their role in regenerative medicine. J. Biomed. Mater. Res. Part B Appl. Biomater. 1–15 (2017). doi: 10.1002/jbm.b.33865.
S. Farnebo, C.Y. Woon, M. Kim, H. Pham, and J. Chang: Reconstruction of the tendon-bone insertion with decellularized tendon-bone composite grafts: comparison with onventional repair. J. Hand Surg. Am. 39, 65–74 (2014).
A.G. Schwartz, J.H. Lipner, J.D. Pasteris, G.M. Genin, and S. Thomopoulos: Muscle loading is necessary for the formation of a functional tendon enthesis. Bone 55, 44–51 (2013).
H. Lin, T.P. Lozito, P.G. Alexander, R. Gottardi, and R.S. Tuan: Stem cell-based microphysiological osteochondral system to model tissue response to interleukin-1 β. Mol. Pharm. 11, 2203–2212 (2014).
W.L. Grayson, S. Bhumiratana, P.H. Grace Chao, C.T. Hung, and G. Vunjak-Novakovic: Spatial regulation of human mesenchymal stem cell differentiation in engineered osteochondral constructs: effects of pre-differentiation, soluble factors and medium perfusion. Osteoarthr. Cartil. 18, 714–723 (2010).
S.M. Goldman and G.A. Barabino: Spatial Engineering of osteochondral tissue constructs through microfluidically directed differentiation of mesenchymal stem cells. Biores. Open Access 5.1, 109–117 (2016).
N. Jaiswal, S.E. Haynesworth, A.I. Caplan, and S.P. Bruder: Osteogenic differentiation of purified, culture-expanded human mesenchymal stem cells in vitro. J. Cell. Biochem. 64, 295–312 (1997).
W.L. Grayson, M. Fröhlich, K. Yeager, S. Bhumiratana, M.E. Chan, C. Cannizzaro, L.Q. Wan, X.S. Liu, X.E. Guo, and G. Vunjak-Novakovic: Engineering anatomically shaped human bone grafts. Proc. Natl. Acad. Sci. USA 107, 3299–3304 (2010).
A.L. Boskey and R. Roy: Cell culture systems for studies of bone and tooth mineralization. Chem. Rev. 108, 4716–4733 (2008).
R-I. Hata and H. Senoo: L-Ascorbic acid 2-phosphate stimulates collagen accumulation, cell proliferation, and formation of a three-dimensional tissue like substance by skin fibroblasts. J. Cell. Physiol. 138, 8–16 (1989).
R.I. Schwarz, P. Kleinman, and N. Owens: Ascorbate can act as an inducer of the collagen pathway because most steps are tightly coupled. Ann. New York Acad. Sci. 498, 172–185 (1987).
Q. Li, F. Qu, B. Han, R. Mauck, L. Han, and D. Ph: Micromechanical heterogeneity and anisotropy of the meniscus extracellular matrix. Acta Biomater. 54, 356–366 (2017).
S.M. Goldman and G.A. Barabino: Cultivation of agarose-based microfluidic hydrogel promotes the development of large, full-thickness, tissue-engineered articular cartilage constructs. J. Tissue Eng. Regen. Med. 11, 572–581 (2014).
S. Thomopoulos, J.P. Marquez, B. Weinberger, V. Birman, and G.M. Genin: Collagen fiber orientation at the tendon to bone insertion and its influence on stress concentrations. J. Biomech. 39, 1842–1851 (2006).
D.F. Villegas, T.A. Hansen, D.F. Liu, and T.L. Haut Donahue: A quantitative study of the microstructure and biochemistry of the medial meniscal horn attachments. Ann. Biomed. Eng. 36, 123–131 (2008).
D.F. Villegas, and T.L. Haut Donahue: Collagen morphology in human meniscal attachments: a SEM study. Connect. Tissue Res. 51, 327–336 (2010).
D.F. Villegas, J.A. Maes, S.D. Magee, and T.L. Haut Donahue: Failure properties and strain distribution analysis of meniscal attachments. J. Biomech. 40, 2655–2662 (2007).
Y. Hu, V. Birman, A. Demyier-Black, A.G. Schwartz, S. Thomopoulos, and G.M. Genin: Stochastic interdigitation as a toughening mechanism at the interface between tendon and bone. Biophys. J. 108, 431–437 (2015).
H.M. Kim, L.M. Galatz, N. Patel, R. Das, and S. Thomopoulos: Recovery potential after postnatal shoulder paralysis. J. Bone Jt. Surg. 91, 879–891 (2009).
S. Thomopoulos, G.R. Williams, and L.J. Soslowsky: Tendon to bone healing: differences in biomechanical, structural, and compositional properties due to a range of activity levels. J. Biomech. Eng. 125, 106 (2003).
C.R. Clark and J.A. Ogden: Prenatal and postnatal development of human knee joint mensci. Iowa Orthop. J. 1, 20–27 (1981).
D. Huang, T.R. Chang, A. Aggarwal, R.C. Lee, H.P. Ehrlich: Mechanisms and dynamics of mechanical strengthening in ligament-equivalent fibroblast-populated collagen matrices. Ann. Biomed. Eng. 21, 289–305 (1993).
S. Thomopoulos, G.M. Fomovsky, and J.W. Holmes: The development of structural and mechanical anisotropy in fibroblast populated collagen gels. J. Biomech. Eng. 127, 742–750 (2005).
F. Grinnell: Fibroblast-collagen-matrix contraction: growth-factor signalling and mechanical loading. Trends Cell Biol. 10, 362–365 (2000).
K.D. Costa, E.J. Lee, and J.W. Holmes: Creating alignment and anisotropy in engineered heart tissue: role of boundary conditions in a model three-dimensional culture system. Tissue Eng. 9, 567–577 (2003).
V.S. Nirmalanandhan, M.S. Levy, A.J. Huth, and D.L. Butler: Effects of cell seeding density and collagen concentration on contraction kinetics of mesenchymal stem cell-seeded collagen constructs. Tissue Eng. 12, 1865–1872 (2006).
R.G. Young, D.L. Butler, W. Weber, A.I. Caplan, S.L. Gordon, and D.J. Fink: Use of mesenchymal stem cells in a collagen matrix for Achilles tendon repair. J. Orthop. Res. 16, 406–413 (1998).
H.A. Awad, D.L. Butler, M.T. Harris, R.E. Ibrahim, Y. Wu, R.G. Young, S. Kadiyala, G.P. Boivin: In vitro characterization of mesenchymal stem cell-seeded collagen scaffolds for tendon repair: effects of initial seeding density on contraction kinetics. J. Biomed. Mater. Res. 51, 233–240 (2000).
R.D. Bowles, R.M. Williams, W.R. Zipfel, and L.J. Bonassar: Self-assembly of aligned tissue-engineered annulus fibrosus and intervertebral disc composite via collagen gel contraction. Tissue Eng. Part A 16, 1339–1348 (2010).
J.L. Puetzer, I. Sallent, A. Gelmi, and M.M. Stevens: Investigating collagen fiber formation for functional musculoskeletal engineering: going beyond the fibril. ORS 2017 Annual Meeting, San Diego, CA, Session No. 50, Vol. 42, Paper No. 348, 2017.
D.J. Huey and K.A. Athanasiou: Tension-compression loading with chemical stimulation results in additive increases to functional properties of anatomic meniscal constructs. PLoS ONE 6, e27857 (2011).
J.L. Puetzer and L.J. Bonassar: Physiologically distributed loading patterns drive the formation of zonally organized collagen structures in tissue engineered meniscus. Tissue Eng. A 22, 907–916 (2016).
C.T. Hendley, J. Tao, J.A. Kunitake, J.J. De Yoreo, and L.A. Estroff: Microscopy techniques for investigating the control of organic constituents on biomineralization. MRS Bull. 40, 480–489 (2015).
H. Wang, A.O. Gee, I.D. Hutchinson, K. Stoner, R.F. Warren, T.O. Chen and S.A. Maher: Bone plug versus suture-only fixation of meniscal grafts: effect on joint contact mechanics during simulated gait. Am. J. Sports Med. 42, 1682–1689 (2014).
K.A. Ross, R.M. Williams, L.V. Schnabel, H.O. Mohammed, H.G. Potter, G. Bradica, E. Castiglione, S.L. Pownder, P.W. Satchell, R.A. Saska, and L.A. Fortier: Comparison of three methods to quantify repair cartilage collagen orientation. Cartilage 4, 111–120 (2013).
N.T. Khanarian, M.K. Boushell, J.P. Spalazzi, N. Pleshko, A.L. Boskey, and H.H. Lu: FTIR-I compositional mapping of the cartilage-to-bone interface as a function of tissue region and age. J. Bone Miner. Res. 29, 1–26 (2014).
J.C. Mansfield, J. Moger, E. Green, C. Moger, and C.P. Winlove: Chemically specific imaging and in-situ chemical analysis of articular cartilage with stimulated Raman scattering. J. Biophotonics 6, 803–814 (2013).
S. Yamanaka: A fresh look at iPS cells. Cell 137, 13–17 (2009).
E.S. Lander: The heroes of CRISPR. Cell 164, 18–28 (2016).
N.W. Choi, M. Cabodi, B. Held, J.P. Gleghorn, L.J. Bonassar, and A.D. Stroock: Microfluidic scaffolds for tissue engineering. Nat. Mater. 6, 908–915 (2007).
R.R. Jose, M.J. Rodriguez, T.A. Dixon, F. Omenetto, and D.L. Kaplan: Evolution of bioinks and additive manufacturing technologies for 3D bioprinting. ACS Biomater. Sci. Eng. 2, 1662–1678 (2016).
Acknowledgments
The authors acknowledge support from the National Center for Advancing Translational Sciences (NCATS) grant TL1TR000459 of the Clinical and Translational Science Center at Weill Cornell Medical College, and A.J.B. acknowledges a pre-doctoral fellowship award (F31AR070009) from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) of the National Institutes of Health (NIH). The authors would like to thank Leanne Iannucci, Benjamin Cohen, and Jongkil Kim for critical reading of the manuscript and Mary Lou Norman for preparing histological sections.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Authors contributed equally to the manuscript.
Rights and permissions
About this article
Cite this article
Boys, A.J., McCorry, M.C., Rodeo, S. et al. Next generation tissue engineering of orthopedic soft tissue-to-bone interfaces. MRS Communications 7, 289–308 (2017). https://doi.org/10.1557/mrc.2017.91
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/mrc.2017.91