Abstract
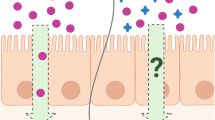
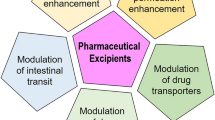
Accumulating evidence from the last decade has shown that many pharmaceutical excipients are not pharmacologically inert but instead have effects on metabolic enzymes and/or drug transporters. Hence, the absorption, distribution, metabolism, and elimination (ADME) of active pharmaceutical ingredients (APIs) may be altered due to the modulation of their metabolism and transport by excipients. The impact of excipients is a potential concern for Biopharmaceutics Classification System (BCS)-based biowaivers, particularly as the BCS-based biowaivers have been extended to class 3 drugs in certain dosage forms. The presence of different excipients or varying amounts of excipients between formulations may result in bio-inequivalence. The excipient impact may lead to significant variations in clinical outcomes as well. The aim of this paper is to review the recent findings of excipient effects on gastrointestinal (GI) absorption, focusing on their interactions with the metabolic enzymes and transporters in the GI tract. A wide range of commonly used excipients such as binders, diluents, fillers, solvents, and surfactants are discussed here. We summarized the reported effects of those excipients on GI tract phase I and phase II enzymes, uptake and efflux transporters, and relevant clinical significance. This information can enhance our understanding of excipient influence on drug absorption and is useful in designing pharmacokinetic studies and evaluating the resultant data.
Similar content being viewed by others
References
Gavhane YN, Yadav AV. Loss of orally administered drugs in GI tract. Saudi Pharm J SPJ: Off Publ Saudi Pharm Soc. 2012;20(4):331–44.
Lipinski CA. Drug-like properties and the causes of poor solubility and poor permeability. J Pharmacol Toxicol Methods. 2000;44(1):235–49.
Bardelmeijer HA, van Tellingen O, Schellens JH, Beijnen JH. The oral route for the administration of cytotoxic drugs: strategies to increase the efficiency and consistency of drug delivery. Investig New Drugs. 2000;18(3):231–41.
Katsura T, Inui K. Intestinal absorption of drugs mediated by drug transporters: mechanisms and regulation. Drug Metab Pharmacokinet. 2003;18(1):1–15.
Lee V, Yamamoto A. Penetration and enzymatic barriers to peptide and protein absorption. Adv Drug Deliv Rev. 1989;4(2).
Crauste-Manciet S, Decroix M, Farinotti R, Chaumeil J. Cefpodoxime-proxetil hydrolysis and food effects in the intestinal lumen before absorption: in vitro comparison of rabbit and human material. Int J Pharm. 1997;157(2):153–61.
Canny GO, McCormick BA. Bacteria in the intestine, helpful residents or enemies from within? Infect Immun. 2008;76(8):3360–73.
Ayman El-Kattan MV. Oral absorption, intestinal metabolism and human oral bioavailability. Paxton J, editor: InTech; 2012.
Chaudhari SP, Patil PS. Pharmaceutical excipients: a review. Int J Adv Pharmacy Biol Chem. 2012;1(1):21–34.
Buggins TR, Dickinson PA, Taylor G. The effects of pharmaceutical excipients on drug disposition. Adv Drug Deliv Rev. 2007;59(15):1482–503.
Shilpa P, Chaudhari PSP. Pharmaceutical excipients: a review. Int J Adv Pharmacy Biol Chem. 2012;1(1):21–34.
Goole J, Lindley DJ, Roth W, Carl SM, Amighi K, Kauffmann JM, et al. The effects of excipients on transporter mediated absorption. Int J Pharm. 2010;393(1–2):17–31.
Paine MF, Hart HL, Ludington SS, Haining RL, Rettie AE, Zeldin DC. The human intestinal cytochrome P450 “pie”. Drug Metab Dispos Biol Fate Chem. 2006;34(5):880–6.
Nebert DW, Russell DW. Clinical importance of the cytochromes P450. Lancet. 2002;360(9340):1155–62.
Lewis DF. 57 varieties: the human cytochromes P450. Pharmacogenomics. 2004;5(3):305–18.
Yan Z, Caldwell GW. Metabolism profiling, and cytochrome P450 inhibition & induction in drug discovery. Curr Top Med Chem. 2001;1(5):403–25.
Mawe GM, Gardette R, D’Agostaro L, Role LW. Development of synaptic transmission at autonomic synapses in vitro revealed by cytochrome oxidase histochemistry. J Neurobiol. 1990;21(4):578–91.
Labroo RB, Paine MF, Thummel KE, Kharasch ED. Fentanyl metabolism by human hepatic and intestinal cytochrome P450 3A4: implications for interindividual variability in disposition, efficacy, and drug interactions. Drug Metab Dispos Biol Fate Chem. 1997;25(9):1072–80.
Lown KS, Ghosh M, Watkins PB. Sequences of intestinal and hepatic cytochrome P450 3A4 cDNAs are identical. Drug Metab Dispos Biol Fate Chem. 1998;26(2):185–7.
Bravo Gonzalez RC, Huwyler J, Boess F, Walter I, Bittner B. In vitro investigation on the impact of the surface-active excipients Cremophor EL, Tween 80 and Solutol HS 15 on the metabolism of midazolam. Biopharm Drug Dispos. 2004;25(1):37–49.
Ren X, Mao X, Cao L, Xue K, Si L, Qiu J, et al. Nonionic surfactants are strong inhibitors of cytochrome P450 3A biotransformation activity in vitro and in vivo. Eur J Pharm Sci Off J Eur Fed Pharm Sci. 2009;36(4–5):401–11.
Christiansen A, Backensfeld T, Denner K, Weitschies W. Effects of non-ionic surfactants on cytochrome P450-mediated metabolism in vitro. Eur J Pharm Biopharm Off J Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik eV. 2011;78(1):166–72.
Ren X, Mao X, Si L, Cao L, Xiong H, Qiu J, et al. Pharmaceutical excipients inhibit cytochrome P450 activity in cell free systems and after systemic administration. Eur J Pharm Biopharm Off J Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik eV. 2008;70(1):279–88.
Huang J, Si L, Jiang L, Fan Z, Qiu J, Li G. Effect of pluronic F68 block copolymer on P-glycoprotein transport and CYP3A4 metabolism. Int J Pharm. 2008;356(1–2):351–3.
Zhu S, Huang R, Hong M, Jiang Y, Hu Z, Liu C, et al. Effects of polyoxyethylene (40) stearate on the activity of P-glycoprotein and cytochrome P450. Eur J Pharm Sci Off J Eur Fed Pharm Sci. 2009;37(5):573–80.
Martin P, Giardiello M, McDonald TO, Rannard SP, Owen A. Mediation of in vitro cytochrome p450 activity by common pharmaceutical excipients. Mol Pharm. 2013;10(7):2739–48.
Tompkins L, Lynch C, Haidar S, Polli J, Wang H. Effects of commonly used excipients on the expression of CYP3A4 in colon and liver cells. Pharm Res. 2010;27(8):1703–12.
Wrighton SA, Ring BJ, Watkins PB, VandenBranden M. Identification of a polymorphically expressed member of the human cytochrome P-450III family. Mol Pharmacol. 1989;36(1):97–105.
Soars MG, Grime K, Riley RJ. Comparative analysis of substrate and inhibitor interactions with CYP3A4 and CYP3A5. Xenobiotica; the fate of foreign compounds in biological systems. 2006;36(4):287–99.
Wrighton SA, Brian WR, Sari MA, Iwasaki M, Guengerich FP, Raucy JL, et al. Studies on the expression and metabolic capabilities of human liver cytochrome P450IIIA5 (HLp3). Mol Pharmacol. 1990;38(2):207–13.
Schuetz EG, Schuetz JD, Strom SC, Thompson MT, Fisher RA, Molowa DT, et al. Regulation of human liver cytochromes P-450 in family 3A in primary and continuous culture of human hepatocytes. Hepatology. 1993;18(5):1254–62.
Wolf KK, Paine MF, Watkins PB. Metabolic barrier of the gastrointestinal tract. Gastrointestinal Toxicology. 10. Second ed 2010. p 53–75.
Wang HJ, Hsiong CH, Ho ST, Lin MJ, Shih TY, Huang PW, et al. Commonly used excipients modulate UDP-glucuronosyltransferase 2B7 activity to improve nalbuphine oral bioavailability in humans. Pharm Res. 2014;31(7):1676–88.
Ro J, Kim H, Shim BH, Kim I, Kim JT, Kim H, et al. In vitro metabolic modulation of aryl sulfotransferases by pharmaceutical excipients. B Korean Chem Soc. 2014;35(8):2577–80.
Jancova P, Anzenbacher P, Anzenbacherova E. Phase Ii drug metabolizing enzymes. Biomed Pap. 2010;154(2):103–16.
Barre L, Fournel-Gigleux S, Finel M, Netter P, Magdalou J, Ouzzine M. Substrate specificity of the human UDP-glucuronosyltransferase UGT2B4 and UGT2B7—identification of a critical aromatic amino acid residue at position 33. FEBS J. 2007;274(5):1256–64.
Negishi M, Pedersen LG, Petrotchenko E, Shevtsov S, Gorokhov A, Kakuta Y, et al. Structure and function of sulfotransferases. Arch Biochem Biophys. 2001;390(2):149–57.
Kusuhara H, Sugiyama Y. Pharmacokinetic modeling of the hepatobiliary transport mediated by cooperation of uptake and efflux transporters. Drug Metab Rev. 2010;42(3):539–50.
Zhang EY, Knipp GT, Ekins S, Swaan PW. Structural biology and function of solute transporters: implications for identifying and designing substrates. Drug Metab Rev. 2002;34(4):709–50.
Szakacs G, Varadi A, Ozvegy-Laczka C, Sarkadi B. The role of ABC transporters in drug absorption, distribution, metabolism, excretion and toxicity (ADME-Tox). Drug Discov Today. 2008;13(9–10):379–93.
Peters SA. Physiologically based pharmacokinetic (PBPK) modeling and simulations: principles, methods, and applications in the pharmaceutical industry. Hoboken: Wiley; 2011. xvii, 430 p.
Locher KP. Review. Structure and mechanism of ATP-binding cassette transporters. Philos Trans R Soc Lond Ser B Biol Sci. 2009;364(1514):239–45.
Chen ML. Lipid excipients and delivery systems for pharmaceutical development: a regulatory perspective. Adv Drug Deliv Rev. 2008;60(6):768–77.
Hanke U, May K, Rozehnal V, Nagel S, Siegmund W, Weitschies W. Commonly used nonionic surfactants interact differently with the human efflux transporters ABCB1 (p-glycoprotein) and ABCC2 (MRP2). Eur J Pharm Biopharm Off J Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik eV. 2010;76(2):260–8.
Zhao G, Huang J, Xue K, Si L, Li G. Enhanced intestinal absorption of etoposide by self-microemulsifying drug delivery systems: roles of P-glycoprotein and cytochrome P450 3A inhibition. Eur J Pharm Sci Off J Eur Fed Pharm Sci. 2013;50(3–4):429–39.
Ma L, Wei Y, Zhou Y, Ma X, Wu X. Effects of Pluronic F68 and Labrasol on the intestinal absorption and pharmacokinetics of rifampicin in rats. Arch Pharm Res. 2011;34(11):1939–43.
Guan Y, Huang J, Zuo L, Xu J, Si L, Qiu J, et al. Effect of pluronic P123 and F127 block copolymer on P-glycoprotein transport and CYP3A metabolism. Arch Pharm Res. 2011;34(10):1719–28.
Shaik N, Giri N, Elmquist WF. Investigation of the micellar effect of pluronic P85 on P-glycoprotein inhibition: cell accumulation and equilibrium dialysis studies. J Pharm Sci. 2009;98(11):4170–90.
Cornaire G, Woodley J, Hermann P, Cloarec A, Arellano C, Houin G. Impact of excipients on the absorption of P-glycoprotein substrates in vitro and in vivo. Int J Pharm. 2004;278(1):119–31.
Dintaman JM, Silverman JA. Inhibition of P-glycoprotein by D-alpha-tocopheryl polyethylene glycol 1000 succinate (TPGS). Pharm Res. 1999;16(10):1550–6.
Bogman K, Erne-Brand F, Alsenz J, Drewe J. The role of surfactants in the reversal of active transport mediated by multidrug resistance proteins. J Pharm Sci. 2003;92(6):1250–61.
Parsa A, Saadati R, Abbasian Z, Azad Aramaki S, Dadashzadeh S. Enhanced permeability of etoposide across everted sacs of rat small intestine by vitamin E-TPGS. Iran J Pharm Res IJPR. 2013;12(Suppl):37–46.
Varma MV, Panchagnula R. Enhanced oral paclitaxel absorption with vitamin E-TPGS: effect on solubility and permeability in vitro, in situ and in vivo. Europ J Pharm Sci Off J Eur Fed Pharm Sci. 2005;25(4–5):445–53.
Bittner B, Guenzi A, Fullhardt P, Zuercher G, Gonzalez RC, Mountfield RJ. Improvement of the bioavailability of colchicine in rats by co-administration of D-alpha-tocopherol polyethylene glycol 1000 succinate and a polyethoxylated derivative of 12-hydroxy-stearic acid. Arzneimittelforschung. 2002;52(9):684–8.
Li W, Li X, Gao Y, Zhou Y, Ma S, Zhao Y, et al. Inhibition mechanism of P-glycoprotein mediated efflux by mPEG-PLA and influence of PLA chain length on P-glycoprotein inhibition activity. Mol Pharm. 2014;11(1):71–80.
Simon S, Schubert R. Inhibitory effect of phospholipids on P-glycoprotein: cellular studies in Caco-2, MDCKII mdr1 and MDCKII wildtype cells and P-gp ATPase activity measurements. Biochimica et Biophysica Acta (BBA) - Mol Cell Biol Lipids. 2012;1821(9):1211–23.
Yu H, Hu YQ, Ip FC, Zuo Z, Han YF, Ip NY. Intestinal transport of bis(12)-hupyridone in Caco-2 cells and its improved permeability by the surfactant Brij-35. Biopharm Drug Dispos. 2011;32(3):140–50.
Ashiru-Oredope DAI, Patel N, Forbes B, Patel R, Basit AW. The effect of polyoxyethylene polymers on the transport of ranitidine in Caco-2 cell monolayers. Int J Pharm. 2011;409(1–2):164–8.
Barta CA, Sachs-Barrable K, Feng F, Wasan KM. Effects of monoglycerides on P-glycoprotein: modulation of the activity and expression in Caco-2 cell monolayers. Mol Pharm. 2008;5(5):863–75.
Mo R, Xiao Y, Sun M, Zhang C, Ping Q. Enhancing effect of N-octyl-O-sulfate chitosan on etoposide absorption. Int J Pharm. 2011;409(1–2):38–45.
Vlaming ML, Lagas JS, Schinkel AH. Physiological and pharmacological roles of ABCG2 (BCRP): recent findings in Abcg2 knockout mice. Adv Drug Deliv Rev. 2009;61(1):14–25.
Yamagata T, Kusuhara H, Morishita M, Takayama K, Benameur H, Sugiyama Y. Effect of excipients on breast cancer resistance protein substrate uptake activity. J Control Release Off J Control Release Soc. 2007;124(1–2):1–5.
Yamagata T, Kusuhara H, Morishita M, Takayama K, Benameur H, Sugiyama Y. Improvement of the oral drug absorption of topotecan through the inhibition of intestinal xenobiotic efflux transporter, breast cancer resistance protein, by excipients. Drug Metab Dispos Biol Fate Chem. 2007;35(7):1142–8.
Yamagata T, Morishita M, Kusuhara H, Takayama K, Benameur H, Sugiyama Y. Characterization of the inhibition of breast cancer resistance protein-mediated efflux of mitoxantrone by pharmaceutical excipients. Int J Pharm. 2009;370(1–2):216–9.
Aspenstrom-Fagerlund B, Tallkvist J, Ilback NG, Glynn AW. Oleic acid decreases BCRP mediated efflux of mitoxantrone in Caco-2 cell monolayers. Food Chem Toxicol Int J Published Br Ind Biol Res Assoc. 2012;50(10):3635–45.
Dietrich CG, Geier A, Oude Elferink RP. ABC of oral bioavailability: transporters as gatekeepers in the gut. Gut. 2003;52(12):1788–95.
Li L, Yi T, Lam CW. Interactions between human multidrug resistance related protein (MRP2; ABCC2) and excipients commonly used in self-emulsifying drug delivery systems (SEDDS). Int J Pharm. 2013;447(1–2):192–8.
Li L, Yi T, Lam CW. Inhibition of human efflux transporter ABCC2 (MRP2) by self-emulsifying drug delivery system: influences of concentration and combination of excipients. J Pharm Pharm Sci Publ Can Soc Pharm Sci Soc Can Sci Pharm. 2014;17(4):447–60.
Jia JX, Wasan KM. Effects of monoglycerides on rhodamine 123 accumulation, estradiol 17 beta-D-glucuronide bidirectional transport and MRP2 protein expression within Caco-2 cells. J Pharm Pharm Sci Publ Can Soc Pharm Sci Soc Can Sci Pharm. 2008;11(3):45–62.
Tamai I. Oral drug delivery utilizing intestinal OATP transporters. Adv Drug Deliv Rev. 2012;64(6):508–14.
Engel A, Oswald S, Siegmund W, Keiser M. Pharmaceutical excipients influence the function of human uptake transporting proteins. Mol Pharm. 2012;9(9):2577–81.
Porter CJ, Trevaskis NL, Charman WN. Lipids and lipid-based formulations: optimizing the oral delivery of lipophilic drugs. Nat Rev Drug Discov. 2007;6(3):231–48.
Houston JB, Levy H. Effect of various alcohols on intestinal net water flux and theophylline absorption in rats. J Pharm Sci. 1975;64.
Ashiru DA, Patel R, Basit AW. Polyethylene glycol 400 enhances the bioavailability of a BCS class III drug (ranitidine) in male subjects but not females. Pharm Res. 2008;25(10):2327–33.
Stavchansky S. Scientific perspectives on extending the provision for waivers of in vivo bioavailability and bioequivalence studies for drug products containing high solubility-low permeability drugs (BCS-Class 3). AAPS J. 2008;10(2):300–5.
Basit AW, Podczeck F, Newton JM, Waddington WA, Ell PJ, Lacey LF. Influence of polyethylene glycol 400 on the gastrointestinal absorption of ranitidine. Pharm Res. 2002;19(9):1368–74.
Schulze JD, Waddington WA, Eli PJ, Parsons GE, Coffin MD, Basit AW. Concentration-dependent effects of polyethylene glycol 400 on gastrointestinal transit and drug absorption. Pharm Res. 2003;20(12):1984–8.
Tomaru A, Takeda-Morishita M, Maeda K, Banba H, Takayama K, Kumagai Y, et al. Effects of Cremophor EL on the absorption of orally administered saquinavir and fexofenadine in healthy subjects. Drug Metab Pharmacokinet. 2015;30(3):221–6.
Vaithianathan S, Haidar SH, Zhang X, Jiang W, Avon C, Dowling TC, et al. Effect of common excipients on the oral drug absorption of biopharmaceutics classification system class 3 drugs cimetidine and acyclovir. J Pharm Sci. 2015:n/a-n/a.
Chen ML, Straughn AB, Sadrieh N, Meyer M, Faustino PJ, Ciavarella AB, et al. A modern view of excipient effects on bioequivalence: case study of sorbitol. Pharm Res. 2007;24(1):73–80.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Guest Editor: Peng Zou
Wenpeng Zhang and Yanyan Li contributed equally to this work.
Rights and permissions
About this article
Cite this article
Zhang, W., Li, Y., Zou, P. et al. The Effects of Pharmaceutical Excipients on Gastrointestinal Tract Metabolic Enzymes and Transporters—an Update. AAPS J 18, 830–843 (2016). https://doi.org/10.1208/s12248-016-9928-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12248-016-9928-8