Abstract:
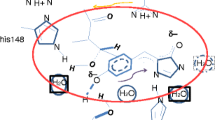
Absorption of gas-phase biomolecules has been studied at the heavy-ion storage ring ELISA. Here we discuss the absorption characteristics of the chromophores of the Green Fluorescent Protein (GFP). The gas-phase absorption maximum of the deprotonated chromophore (anion form) is at 479 nm. This is almost identical to one of the two absorption maxima of the protein, being at 477 nm, which is ascribed to a deprotonated chromophore in the protein. The protonated chromophore (cation form) has a maximum at 406 nm in the gas phase. We compare the gas-phase results with absorption profiles of GFP and chromophores in liquids, and argue that the absorption characteristics of GFP are mainly ascribed to intrinsic chemical properties of the chromophore. Evidently, the special β-can structure of GFP provides shielding of the chromophore from the surroundings without significantly changing the electronic structure of the chromophore through interactions with amino acid side chains.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received 28 December 2001 Published online 13 September 2002
Rights and permissions
About this article
Cite this article
Andersen, L., Lapierre, A., Nielsen, S. et al. Chromophores of the green fluorescent protein studied in the gas phase. Eur. Phys. J. D 20, 597–600 (2002). https://doi.org/10.1140/epjd/e2002-00141-0
Issue Date:
DOI: https://doi.org/10.1140/epjd/e2002-00141-0