Abstract
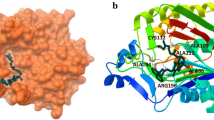
A series of novel 1,2,4-triazole nonsteroidal anti-inflammatory drugs (NSAIDs) derived from etodolac were designed and synthesized. The synthesized compounds were identified using 1H and 13C NMR, IR, and mass spectra and elemental analyses and evaluated for their in vitro antibacterial activity against Gram-positive microorganisms like Streptococcus pneumoniae and Klebsiella pneumoniae and Gram-negative strains such as Pseudomonas aeruginosa and Enterobacter cloacae with pefloxacin as a reference drug. Some compounds showed a potent activity at a concentration 50 mg/mL with inhibition zones of 30 to 36 mm against S. pneumoniae. Etodolac-derived N-isobutyl- and N-ethyl-1,2,4-triazoles containing 4-methoxybenzylsulfanyl and 3-nitrobenzylsulfanyl groups were active against P. aeruginosa with inhibition zones of 25–29 mm at a concentration of 50 mg/mL. All compounds showed excellent antioxidant activity with IC50 values ranging from 72.39±0.25 µg/mL to 16.39±0.65 µg/mL in comparison with ascorbic acid (IC50 16.81±0.18 µg/mL). Molecular docking studies revealed strong hydrogen bonding, π–π, and π–σ interactions of 3-nitro-, 4-methoxy-, and 4-methylbenzyl moieties with Ser421, Val120, Tyr124, Phe319, Ala44, and Val120 amino acid residues of the active site of glycogen synthase kinase-3 (GSK-3) protein.


Similar content being viewed by others
REFERENCES
Holla, B.S., Mahalinga, M., Karthikeyen, M.S., Poojary, B., Akberali, P.M., and Kumari, N.S., Eur. J. Med. Chem., 2005, vol. 40, p. 1173. https://doi.org/10.1016/j.ejmech.2005.02.013
Sheremet, E.A., Tomanov, R.I., Trukhin, E.V., and Berestovitskaya, V.M., Russ. J. Org. Chem., 2004, vol. 40, p. 594. https://doi.org/10.1023/B:RUJO.0000036090.61432.18
Hafez, H.N., Abbas, H.A, and El-Gazzar, A.R., Acta Pharm., 2008, vol. 58, p. 359. https://doi.org/10.2478/v10007-008-0024-1
Banu, K.M., Dinaker, A., and Ananthnarayan, C., Indian J. Pharm. Sci., 1999, vol. 61, p. 202.
Guan, L.P., Jin, Q.H., Tian, G.R., Chai, K.Y., and Quan, Z.S., J. Pharm. Pharm. Sci., 2007, vol. 10, p. 254.
Passannanti, A., Diana, P., Barraja, P., Mingooia, F., Lauria, A., and Cirrincine, G., Heterocycles, 1998, vol. 48, p. 1229. https://doi.org/10.3987/COM-98-8130
Gujjar, R., Marwaha, A., White, J., White, L., Creason, S., Shackleford, D.M., Baldwin, J., Charman, W.N., Buckner, F.S., Charman, S., Rathod, P.K., and Phillips, M.A., J. Med. Chem., 2009, vol. 52, p. 1864. https://doi.org/10.1021/jm801343r
Johns, B.A., Weatherhead, J.G., Allen, S.H., Thompson, J.B., Garvey, E.P., and Foster, S.A., Bioorg. Med. Chem. Lett., 2009, vol. 19, p. 1802. https://doi.org/10.1016/j.bmcl.2009.01.090
Manfredini, S., Vicentini, C.B., Manfrini, M., Bianchi, N., Rustigliano, C., Mischiati, C., and Gambari, R., Bioorg. Med. Chem., 2000, vol. 8, p. 2343. https://doi.org/10.1016/S0968-0896(00)00160-7
Duran, A., Dogan, H.N., and Rollas, H., Farmaco, 2002, vol. 57, p. 559. https://doi.org/10.1016/S0014-827X(02)01248-X
Sztanke, K., Tuzimski, T., and Rzymowska, J., Eur. J. Med. Chem., 2008, vol. 43, p. 404. https://doi.org/10.1016/j.ejmech.2007.03.033
Liu, P., Zhu, S., and Xie, W., Bioorg. Med. Chem. Lett., 2008, vol. 18, p. 3261. https://doi.org/10.1016/j.bmcl.2008.04.056
El-Sherief, H.A., Abuo-Rahma, G.E.D.A., Shoman, M.E., Beshr, E.A., and Abdelbaky, R.M., Med. Chem. Res., 2017, vol. 26, p. 3077. https://doi.org/10.1007/s00044-017-2004-9
El Shehry, M., Abu-Hashem, A., and El-Telbani, E., Eur. J. Med. Chem., 2010, vol. 45, p. 1906. https://doi.org/10.1016/j.ejmech.2010.01.030
Mathew, V., Keshavayya, J., Vaidya, V., and Giles, D., Eur. J. Med. Chem., 2007, vol. 42, p. 823. https://doi.org/10.1016/j.ejmech.2006.12.010
Holla, B.S., Poojary, K.N., Rao, B.S., and Shivananda, M., Eur. J. Med. Chem., 2002, vol. 37, p. 511. https://doi.org/10.1016/s0223-5234(02)01358-2
Padmavathi, V., Reddy, G.S., Padmaja, A., and Kondaiah, P., Eur. J. Med. Chem., 2009, vol. 44, p. 2106. https://doi.org/10.1016/j.ejmech.2008.10.012
Ashok, D., Rangu, K., Rao, V.H., Gundu, S., Srilata, B., and Vijjulatha, M., Med. Chem. Res., 2016, vol. 25, p. 501. https://doi.org/10.1007/s00044-016-1505-2
Ashok, D., Gundu, S., Aamate, V.K., Devulapally, M.G., Bathiniand, R., and Manga, V., J. Mol. Struct., 2018, vol. 1157, p. 312. https://doi.org/10.1016/j.molstruc.2017.12.080
Adamski, A., Kruszka, D., Dutkiewicz, Z., Kubicki, M., Gorczynski, A., and Patroniak, V., Tetrahedron, 2017, vol. 73, p. 3377. https://doi.org/10.1016/j.tet.2017.05.015
Mobaraki, N., Hemmateenejad, B., Weikl, T.R., and Sakhteman, A., J. Mol. Graphics Modell., 2019, vol. 91, p. 186. https://doi.org/10.1016/j.jmgm.2019.06.011
Analytical Microbiology, Kavanagh, F.W., Ed., New York: Academic, 1972, vol. 2.
Barry, H., Drugs, 1991, vol. 42, p. 569. https://doi.org/10.2165/00003495-199142040-00003
Klein, S.M., Cohen, G., and Cederbaum, A.I., Biochemistry, 1981, vol. 20, p. 6006. https://doi.org/10.1021/bi00524a013
Friesner, R.A., Banks, J.L., Murphy, R.B., Halgren, T.A., Klicic, J.J., Mainz, D.T., Repasky, M.P., Knoll, E.H., Shelley, M., Perry, J.K., Shaw, D.E., Francis, P., and Shenkin, P.S., J. Med. Chem., 2004, vol. 47, p. 1739. https://doi.org/10.1021/jm0306430
ACKNOWLEDGMENTS
A. Shaik thanks our Research Supervisor Pilli V.V.N. Kishore for providing us required facilities and motivation for completion of the research work. We also extend our gratitude toward Department of Sciences and Humanities, VFSTR University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare no conflict of interest.
Supplementary information
Rights and permissions
About this article
Cite this article
Shaik, A., Rao, A.T., Venkatarao, D.V. et al. Novel Etodolac-Based 1,2,4-Triazole Derivatives as Antimicrobial Agents: Synthesis, Biological Evaluation, and Molecular Docking Study. Russ J Org Chem 56, 2179–2187 (2020). https://doi.org/10.1134/S1070428020120210
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070428020120210