Abstract
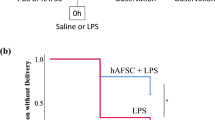
Cell-free fetal DNA in the maternal circulation has been associated with the onset of labor at term. Moreover, clinical studies have suggested that cell-free fetal DNA has value to predict pregnancy complications such as spontaneous preterm labor leading to preterm birth. However, a mechanistic link between cell-free fetal DNA and preterm labor and birth has not been established. Herein, using an allogeneic mouse model in which a paternal green fluorescent protein (GFP) can be tracked in the fetuses, we established that cell-free fetal DNA (Egfp) concentrations were higher in late gestation compared to mid-pregnancy and were maintained at increased levels during the onset of labor at term, followed by a rapid decrease after birth. A positive correlation between cell-free fetal DNA concentrations and the number of GFP-positive pups was also observed. The increase in cell-free fetal DNA concentrations prior to labor at term was not linked to a surge in any specific cytokine/chemokine; yet, specific chemokines (i.e., CCL2, CCL7, and CXCL2) increased as gestation progressed and maintained elevated levels in the postpartum period. In addition, cell-free fetal DNA concentrations increased prior to systemic inflammation-induced preterm birth, which was associated with a strong cytokine response in the maternal circulation. However, cell-free fetal DNA concentrations were not increased prior to intra-amniotic inflammation-induced preterm birth, but in this model, a mild inflammatory response was observed in the maternal circulation. Collectively, these findings suggest that an elevation in cell-free fetal DNA concentrations in the maternal circulation precedes the physiological process of labor at term and the pathological process of preterm labor linked with systemic inflammation, but not that associated with intra-amniotic inflammation.





Similar content being viewed by others
References
Liggins CG. Cervical ripening as an inflammatory reaction. In: Elwood DA, Andersson ABM, editors. Cervix in Pregnancy and Labour. Edinburgh: Churchill Livingstone; 1981. p. 1–9.
Romero R, Brody DT, Oyarzun E, et al. Infection and labor. III. Interleukin-1: a signal for the onset of parturition. Am J Obstet Gynecol. 1989;160(5 Pt 1):1117–23.
Romero R, Gotsch F, Pineles B, Kusanovic JP. Inflammation in pregnancy: its roles in reproductive physiology, obstetrical complications, and fetal injury. Nutr Rev. 2007;65(12 Pt 2):S194–202.
Norman JE, Bollapragada S, Yuan M, Nelson SM. Inflammatory pathways in the mechanism of parturition. BMC Pregnancy Childbirth. 2007;7(Suppl 1):S7.
Gomez-Lopez N, Guilbert LJ, Olson DM. Invasion of the leukocytes into the fetal-maternal interface during pregnancy. J Leukoc Biol. 2010;88(4):625–33.
Norwitz ER, Bonney EA, Snegovskikh VV, et al. Molecular regulation of parturition: the role of the decidual clock. Cold Spring Harb Perspect Med. 2015;5(11).
Bokstrom H, Brannstrom M, Alexandersson M, Norstrom A. Leukocyte subpopulations in the human uterine cervical stroma at early and term pregnancy. Hum Reprod. 1997;12(3):586–90.
Mackler AM, Iezza G, Akin MR, McMillan P, Yellon SM. Macrophage trafficking in the uterus and cervix precedes parturition in the mouse. Biol Reprod. 1999;61(4):879–83.
Young A, Thomson AJ, Ledingham M, Jordan F, Greer IA, Norman JE. Immunolocalization of proinflammatory cytokines in myometrium, cervix, and fetal membranes during human parturition at term. Biol Reprod. 2002;66(2):445–9.
Kelly RW. Inflammatory mediators and cervical ripening. J Reprod Immunol. 2002;57(1-2):217–24.
Osman I, Young A, Ledingham MA, Thomson AJ, Jordan F, Greer IA, et al. Leukocyte density and pro-inflammatory cytokine expression in human fetal membranes, decidua, cervix and myometrium before and during labour at term. Mol Hum Reprod. 2003;9(1):41–5.
Yellon SM, Mackler AM, Kirby MA. The role of leukocyte traffic and activation in parturition. J Soc Gynecol Investig. 2003;10(6):323–38.
Sakamoto Y, Moran P, Bulmer JN, Searle RF, Robson SC. Macrophages and not granulocytes are involved in cervical ripening. J Reprod Immunol. 2005;66(2):161–73.
Yellon SM, Ebner CA, Sugimoto Y. Parturition and recruitment of macrophages in cervix of mice lacking the prostaglandin F receptor. Biol Reprod. 2008;78(3):438–44.
Timmons BC, Fairhurst AM, Mahendroo MS. Temporal changes in myeloid cells in the cervix during pregnancy and parturition. J Immunol. 2009;182(5):2700–7.
Yellon SM, Oshiro BT, Chhaya TY, et al. Remodeling of the cervix and parturition in mice lacking the progesterone receptor B isoform. Biol Reprod. 2011;85(3):498–502.
Clyde LA, Lechuga TJ, Ebner CA, Burns AE, Kirby MA, Yellon SM. Transection of the pelvic or vagus nerve forestalls ripening of the cervix and delays birth in rats. Biol Reprod. 2011;84(3):587–94.
Payne KJ, Clyde LA, Weldon AJ, Milford TA, Yellon SM. Residency and activation of myeloid cells during remodeling of the prepartum murine cervix. Biol Reprod. 2012;87(5):106.
Myers DA. The recruitment and activation of leukocytes into the immune cervix: further support that cervical remodeling involves an immune and inflammatory mechanism. Biol Reprod. 2012;87(5):107.
Thomson AJ, Telfer JF, Young A, Campbell S, Stewart CJ, Cameron IT, et al. Leukocytes infiltrate the myometrium during human parturition: further evidence that labour is an inflammatory process. Hum Reprod. 1999;14(1):229–36.
Shynlova O, Tsui P, Dorogin A, Lye SJ. Monocyte chemoattractant protein-1 (CCL-2) integrates mechanical and endocrine signals that mediate term and preterm labor. J Immunol. 2008;181(2):1470–9.
Shynlova O, Tsui P, Jaffer S, Lye SJ. Integration of endocrine and mechanical signals in the regulation of myometrial functions during pregnancy and labour. Eur J Obstet Gynecol Reprod Biol. 2009;144(Suppl 1):S2–10.
Shynlova O, Lee YH, Srikhajon K, Lye SJ. Physiologic uterine inflammation and labor onset: integration of endocrine and mechanical signals. Reprod Sci. 2013;20(2):154–67.
Lombardi A, Makieva S, Rinaldi SF, Arcuri F, Petraglia F, Norman JE. Expression of matrix metalloproteinases in the mouse uterus and human myometrium during pregnancy, labor, and preterm labor. Reprod Sci. 2018;25(6):938–49.
Fidel PL Jr, Romero R, Ramirez M, et al. Interleukin-1 receptor antagonist (IL-1ra) production by human amnion, chorion, and decidua. Am J Reprod Immunol. 1994;32(1):1–7.
Keelan JA, Marvin KW, Sato TA, Coleman M, McCowan LM, Mitchell MD. Cytokine abundance in placental tissues: evidence of inflammatory activation in gestational membranes with term and preterm parturition. Am J Obstet Gynecol. 1999;181(6):1530–6.
Lonergan M, Aponso D, Marvin KW, Helliwell RJ, Sato TA, Mitchell MD, et al. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL), TRAIL receptors, and the soluble receptor osteoprotegerin in human gestational membranes and amniotic fluid during pregnancy and labor at term and preterm. J Clin Endocrinol Metab. 2003;88(8):3835–44.
Esplin MS, Peltier MR, Hamblin S, Smith S, Fausett MB, Dildy GA, et al. Monocyte chemotactic protein-1 expression is increased in human gestational tissues during term and preterm labor. Placenta. 2005;26(8-9):661–71.
Gomez-Lopez N, Estrada-Gutierrez G, Jimenez-Zamudio L, Vega-Sanchez R, Vadillo-Ortega F. Fetal membranes exhibit selective leukocyte chemotaxic activity during human labor. J Reprod Immunol. 2009;80(1-2):122–31.
Gomez-Lopez N, Vadillo-Perez L, Hernandez-Carbajal A, Godines-Enriquez M, Olson DM, Vadillo-Ortega F. Specific inflammatory microenvironments in the zones of the fetal membranes at term delivery. Am J Obstet Gynecol. 2011;205(3):235 e215–24.
Gomez-Lopez N, Vadillo-Perez L, Nessim S, Olson DM, Vadillo-Ortega F. Choriodecidua and amnion exhibit selective leukocyte chemotaxis during term human labor. Am J Obstet Gynecol. 2011;204(4):364 e369–16.
Hadley EE, Sheller-Miller S, Saade G, et al. Amnion epithelial cell-derived exosomes induce inflammatory changes in uterine cells. Am J Obstet Gynecol. 2018;219(5):478 e471–21.
Vince GS, Starkey PM, Jackson MC, Sargent IL, Redman CW. Flow cytometric characterisation of cell populations in human pregnancy decidua and isolation of decidual macrophages. J Immunol Methods. 1990;132(2):181–9.
Keski-Nisula L, Aalto ML, Katila ML, Kirkinen P. Intrauterine inflammation at term: a histopathologic study. Hum Pathol. 2000;31(7):841–6.
Hamilton S, Oomomian Y, Stephen G, Shynlova O, Tower CL, Garrod A, et al. Macrophages infiltrate the human and rat decidua during term and preterm labor: evidence that decidual inflammation precedes labor. Biol Reprod. 2012;86(2):39.
Gomez-Lopez N, Vega-Sanchez R, Castillo-Castrejon M, Romero R, Cubeiro-Arreola K, Vadillo-Ortega F. Evidence for a role for the adaptive immune response in human term parturition. Am J Reprod Immunol. 2013;69(3):212–30.
Hamilton SA, Tower CL, Jones RL. Identification of chemokines associated with the recruitment of decidual leukocytes in human labour: potential novel targets for preterm labour. PLoS One. 2013;8(2):e56946.
Castillo-Castrejon M, Meraz-Cruz N, Gomez-Lopez N, et al. Choriodecidual cells from term human pregnancies show distinctive functional properties related to the induction of labor. Am J Reprod Immunol. 2014;71(1):86–93.
Dudley DJ, Collmer D, Mitchell MD, Trautman MS. Inflammatory cytokine mRNA in human gestational tissues: implications for term and preterm labor. J Soc Gynecol Investig. 1996;3(6):328–35.
Ammala M, Nyman T, Salmi A, Rutanen EM. The interleukin-1 system in gestational tissues at term: effect of labour. Placenta. 1997;18(8):717–23.
Haddad R, Tromp G, Kuivaniemi H, et al. Human spontaneous labor without histologic chorioamnionitis is characterized by an acute inflammation gene expression signature. Am J Obstet Gynecol. 2006;195(2):394 e391–24.
Hassan SS, Romero R, Haddad R, Hendler I, Khalek N, Tromp G, et al. The transcriptome of the uterine cervix before and after spontaneous term parturition. Am J Obstet Gynecol. 2006;195(3):778–86.
Hassan SS, Romero R, Tarca AL, et al. Signature pathways identified from gene expression profiles in the human uterine cervix before and after spontaneous term parturition. Am J Obstet Gynecol. 2007;197(3):250.e251–7.
Nhan-Chang CL, Romero R, Tarca AL, et al. Characterization of the transcriptome of chorioamniotic membranes at the site of rupture in spontaneous labor at term. Am J Obstet Gynecol. 2010;202(5):462.e461–41.
Mittal P, Romero R, Tarca AL, Gonzalez J, Draghici S, Xu Y, et al. Characterization of the myometrial transcriptome and biological pathways of spontaneous human labor at term. J Perinat Med. 2010;38(6):617–43.
Chaemsaithong P, Madan I, Romero R, Than NG, Tarca AL, Draghici S, et al. Characterization of the myometrial transcriptome in women with an arrest of dilatation during labor. J Perinat Med. 2013;41(6):665–81.
Romero R, Tarca AL, Chaemsaithong P, et al. Transcriptome interrogation of human myometrium identifies differentially expressed sense-antisense pairs of protein-coding and long non-coding RNA genes in spontaneous labor at term. J Matern Fetal Neonatal Med. 2014;27(14):1397–408.
Stephen GL, Lui S, Hamilton SA, et al. Transcriptomic profiling of human choriodecidua during term labor: inflammation as a key driver of labor. Am J Reprod Immunol. 2015;73(1):36–55.
Bukowski R, Sadovsky Y, Goodarzi H, et al. Onset of human preterm and term birth is related to unique inflammatory transcriptome profiles at the maternal fetal interface. PeerJ. 2017;5:e3685.
Arenas-Hernandez M, Gomez-Lopez N, Garcia-Flores V, et al. Choriodecidual leukocytes display a unique gene expression signature in spontaneous labor at term. Genes Immun. 2019;20(1):56–68.
Sacks GP, Studena K, Sargent K, Redman CW. Normal pregnancy and preeclampsia both produce inflammatory changes in peripheral blood leukocytes akin to those of sepsis. Am J Obstet Gynecol. 1998;179(1):80–6.
Naccasha N, Gervasi MT, Chaiworapongsa T, Berman S, Yoon BH, Maymon E, et al. Phenotypic and metabolic characteristics of monocytes and granulocytes in normal pregnancy and maternal infection. Am J Obstet Gynecol. 2001;185(5):1118–23.
Unal ER, Cierny JT, Roedner C, Newman R, Goetzl L. Maternal inflammation in spontaneous term labor. Am J Obstet Gynecol. 2011;204(3):223 e221–5.
Cierny JT, Unal ER, Flood P, et al. Maternal inflammatory markers and term labor performance. Am J Obstet Gynecol. 2014;210(5):447 e441–6.
Neal JL, Lamp JM, Lowe NK, Gillespie SL, Sinnott LT, McCarthy DO. Differences in inflammatory markers between nulliparous women admitted to hospitals in preactive vs active labor. Am J Obstet Gynecol. 2015;212(1):68 e61–8.
Aghaeepour N, Lehallier B, Baca Q, et al. A proteomic clock of human pregnancy. Am J Obstet Gynecol. 2018;218(3):347 e341–14.
Tarca AL, Romero R, Xu Z, et al. Targeted expression profiling by RNA-Seq improves detection of cellular dynamics during pregnancy and identifies a role for T cells in term parturition. Sci Rep. 2019;9(1):848.
Phillippe M. Cell-free fetal DNA--a trigger for parturition. N Engl J Med. 2014;370(26):2534–6.
Phillippe M. Cell-free fetal DNA, telomeres, and the spontaneous onset of parturition. Reprod Sci. 2015;22(10):1186–201.
Herrera CA, Stoerker J, Carlquist J, et al. Cell-free DNA, inflammation, and the initiation of spontaneous term labor. Am J Obstet Gynecol. 2017;217(5):583 e581–8.
Phillippe M. The link between cell-free DNA, inflammation and the initiation of spontaneous labor at term. Am J Obstet Gynecol. 2017;217(5):501–2.
Goldfarb IT, Adeli S, Berk T, Phillippe M. Fetal and placental DNA stimulation of TLR9: a mechanism possibly contributing to the pro-inflammatory events during parturition. Reprod Sci. 2018;25(5):788–96.
Lo YM, Corbetta N, Chamberlain PF, Rai V, Sargent IL, Redman CW, et al. Presence of fetal DNA in maternal plasma and serum. Lancet. 1997;350(9076):485–7.
Lo YM, Tein MS, Lau TK, et al. Quantitative analysis of fetal DNA in maternal plasma and serum: implications for noninvasive prenatal diagnosis. Am J Hum Genet. 1998;62(4):768–75.
Lo YM, Zhang J, Leung TN, Lau TK, Chang AM, Hjelm NM. Rapid clearance of fetal DNA from maternal plasma. Am J Hum Genet. 1999;64(1):218–24.
Ariga H, Ohto H, Busch MP, Imamura S, Watson R, Reed W, et al. Kinetics of fetal cellular and cell-free DNA in the maternal circulation during and after pregnancy: implications for noninvasive prenatal diagnosis. Transfusion. 2001;41(12):1524–30.
Chan LY, Leung TN, Chan KC, Tai HL, Lau TK, Wong EM, et al. Serial analysis of fetal DNA concentrations in maternal plasma in late pregnancy. Clin Chem. 2003;49(4):678–80.
Chan KC, Zhang J, Hui AB, et al. Size distributions of maternal and fetal DNA in maternal plasma. Clin Chem. 2004;50(1):88–92.
Bischoff FZ, Lewis DE, Simpson JL. Cell-free fetal DNA in maternal blood: kinetics, source and structure. Hum Reprod Update. 2005;11(1):59–67.
Birch L, English CA, O'Donoghue K, Barigye O, Fisk NM, Keer JT. Accurate and robust quantification of circulating fetal and total DNA in maternal plasma from 5 to 41 weeks of gestation. Clin Chem. 2005;51(2):312–20.
Majer S, Bauer M, Magnet E, Strele A, Giegerl E, Eder M, et al. Maternal urine for prenatal diagnosis--an analysis of cell-free fetal DNA in maternal urine and plasma in the third trimester. Prenat Diagn. 2007;27(13):1219–23.
Wang E, Batey A, Struble C, Musci T, Song K, Oliphant A. Gestational age and maternal weight effects on fetal cell-free DNA in maternal plasma. Prenat Diagn. 2013;33(7):662–6.
Taglauer ES, Wilkins-Haug L, Bianchi DW. Review: cell-free fetal DNA in the maternal circulation as an indication of placental health and disease. Placenta. 2014;35(Suppl):S64–8.
Jimenez DF, Tarantal AF. Quantitative analysis of male fetal DNA in maternal serum of gravid rhesus monkeys (Macaca mulatta). Pediatr Res. 2003;53(1):18–23.
Khosrotehrani K, Wataganara T, Bianchi DW, Johnson KL. Fetal cell-free DNA circulates in the plasma of pregnant mice: relevance for animal models of fetomaternal trafficking. Hum Reprod. 2004;19(11):2460–4.
Mitsunaga F, Ueiwa M, Kamanaka Y, Morimoto M, Nakamura S. Fetal sex determination of macaque monkeys by a nested PCR using maternal plasma. Exp Anim. 2010;59(2):255–60.
Kadivar A, Hassanpour H, Mirshokraei P, Azari M, Gholamhosseini K, Karami A. Detection and quantification of cell-free fetal DNA in ovine maternal plasma; use it to predict fetal sex. Theriogenology. 2013;79(6):995–1000.
Munoz-Hernandez R, Medrano-Campillo P, Miranda ML, et al. Total and fetal circulating cell-free DNA, angiogenic, and antiangiogenic factors in preeclampsia and HELLP syndrome. Am J Hypertens. 2017;30(7):673–82.
Contro E, Bernabini D, Farina A. Cell-free fetal DNA for the prediction of pre-eclampsia at the first and second trimesters: a systematic review and meta-analysis. Mol Diagn Ther. 2017;21(2):125–35.
Rafaeli-Yehudai T, Imterat M, Douvdevani A, Tirosh D, Benshalom-Tirosh N, Mastrolia SA, et al. Maternal total cell-free DNA in preeclampsia and fetal growth restriction: evidence of differences in maternal response to abnormal implantation. PLoS One. 2018;13(7):e0200360.
Rolnik DL, da Silva Costa F, Lee TJ, Schmid M, McLennan AC. Association between fetal fraction on cell-free DNA testing and first trimester markers for pre-eclampsia. Ultrasound Obstet Gynecol. 2018;52(6):722–727.
Morano D, Rossi S, Lapucci C, Pittalis MC, Farina A. Cell-free DNA (cfDNA) fetal fraction in early- and late-onset fetal growth restriction. Mol Diagn Ther. 2018;22(5):613–9.
Leung TN, Zhang J, Lau TK, Hjelm NM, Lo YM. Maternal plasma fetal DNA as a marker for preterm labour. Lancet. 1998;352(9144):1904–5.
Farina A, LeShane ES, Romero R, Gomez R, Chaiworapongsa T, Rizzo N, et al. High levels of fetal cell-free DNA in maternal serum: a risk factor for spontaneous preterm delivery. Am J Obstet Gynecol. 2005;193(2):421–5.
Jakobsen TR, Clausen FB, Rode L, Dziegiel MH, Tabor A. High levels of fetal DNA are associated with increased risk of spontaneous preterm delivery. Prenat Diagn. 2012;32(9):840–5.
Scharfe-Nugent A, Corr SC, Carpenter SB, et al. TLR9 provokes inflammation in response to fetal DNA: mechanism for fetal loss in preterm birth and preeclampsia. J Immunol. 2012;188(11):5706–12.
Quezada MS, Francisco C, Dumitrascu-Biris D, Nicolaides KH, Poon LC. Fetal fraction of cell-free DNA in maternal plasma in the prediction of spontaneous preterm delivery. Ultrasound Obstet Gynecol. 2015;45(1):101–5.
Dugoff L, Barberio A, Whittaker PG, Schwartz N, Sehdev H, Bastek JA. Cell-free DNA fetal fraction and preterm birth. Am J Obstet Gynecol. 2016;215(2):231 e231–7.
Bauer M, Hutterer G, Eder M, et al. A prospective analysis of cell-free fetal DNA concentration in maternal plasma as an indicator for adverse pregnancy outcome. Prenat Diagn. 2006;26(9):831–6.
Illanes S, Gomez R, Fornes R, Figueroa-Diesel H, Schepeler M, Searovic P, et al. Free fetal DNA levels in patients at risk of preterm labour. Prenat Diagn. 2011;31(11):1082–5.
Stein W, Muller S, Gutensohn K, Emons G, Legler T. Cell-free fetal DNA and adverse outcome in low risk pregnancies. Eur J Obstet Gynecol Reprod Biol. 2013;166(1):10–3.
Poon LC, Musci T, Song K, Syngelaki A, Nicolaides KH. Maternal plasma cell-free fetal and maternal DNA at 11-13 weeks' gestation: relation to fetal and maternal characteristics and pregnancy outcomes. Fetal Diagn Ther. 2013;33(4):215–23.
Thurik FF, Lamain-de Ruiter M, Javadi A, Kwee A, Woortmeijer H, Page-Christiaens GC, et al. Absolute first trimester cell-free DNA levels and their associations with adverse pregnancy outcomes. Prenat Diagn. 2016;36(12):1104–11.
Thaxton JE, Romero R, Sharma S. TLR9 activation coupled to IL-10 deficiency induces adverse pregnancy outcomes. J Immunol. 2009;183(2):1144–54.
Sun Y, Qin X, Shan B, Wang W, Zhu Q, Sharma S, et al. Differential effects of the CpG-Toll-like receptor 9 axis on pregnancy outcome in nonobese diabetic mice and wild-type controls. Fertil Steril. 2013;99(6):1759–67.
Pan D, Gunther R, Duan W, et al. Biodistribution and toxicity studies of VSVG-pseudotyped lentiviral vector after intravenous administration in mice with the observation of in vivo transduction of bone marrow. Mol Ther. 2002;6(1):19–29.
Fidel PL Jr, Romero R, Wolf N, et al. Systemic and local cytokine profiles in endotoxin-induced preterm parturition in mice. Am J Obstet Gynecol. 1994;170(5 Pt 1):1467–75.
Bizargity P, Del Rio R, Phillippe M, Teuscher C, Bonney EA. Resistance to lipopolysaccharide-induced preterm delivery mediated by regulatory T cell function in mice. Biol Reprod. 2009;80(5):874–81.
Cardenas I, Mor G, Aldo P, et al. Placental viral infection sensitizes to endotoxin-induced pre-term labor: a double hit hypothesis. Am J Reprod Immunol. 2011;65(2):110–7.
Roy-Lacroix ME, Guerard M, Berthiaume M, Rola-Pleszczynski M, Crous-Tsanaclis AM, Pasquier JC. Time-dependent effect of in utero inflammation: a longitudinal study in rats. J Matern Fetal Neonatal Med. 2013;26(8):789–94.
Evangelinakis NE, Polyzou EN, Salamalekis GE, Kotsaki AJ, Chrelias CG, Giamarellos-Bourboulis EJ, et al. Alterations in the cellular component of the maternal immune system in a murine preterm delivery model. J Matern Fetal Neonatal Med. 2013;26(10):1024–9.
Gharedaghi MH, Javadi-Paydar M, Yousefzadeh-Fard Y, Salehi-Sadaghiani M, Javadian P, Fakhraei N, et al. Muscimol delays lipopolysaccharide-induced preterm delivery in mice: role of GABA(A) receptors and nitric oxide. J Matern Fetal Neonatal Med. 2013;26(1):36–43.
Shynlova O, Dorogin A, Li Y, Lye S. Inhibition of infection-mediated preterm birth by administration of broad spectrum chemokine inhibitor in mice. J Cell Mol Med. 2014;18(9):1816–29.
Toyama RP, Xikota JC, Schwarzbold ML, et al. Dose-dependent sickness behavior, abortion and inflammation induced by systemic LPS injection in pregnant mice. J Matern Fetal Neonatal Med. 2015;28(4):426–30.
Arenas-Hernandez M, Romero R, St Louis D, Hassan SS, Kaye EB, Gomez-Lopez N. An imbalance between innate and adaptive immune cells at the maternal-fetal interface occurs prior to endotoxin-induced preterm birth. Cell Mol Immunol. 2016;13(4):462–73.
Xu Y, Romero R, Miller D, et al. An M1-like macrophage polarization in decidual tissue during spontaneous preterm labor that is attenuated by rosiglitazone treatment. J Immunol. 2016;196(6):2476–91.
Gomez-Lopez N, Romero R, Arenas-Hernandez M, et al. Intra-amniotic administration of lipopolysaccharide induces spontaneous preterm labor and birth in the absence of a body temperature change. J Matern Fetal Neonatal Med. 2018;31(4):439–46.
Garcia-Flores V, Romero R, Miller D, et al. Inflammation-induced adverse pregnancy and neonatal outcomes can be improved by the immunomodulatory peptide exendin-4. Front Immunol. 2018;9:1291.
Gotsch F, Romero R, Espinoza J, Kusanovic JP, Mazaki-Tovi S, Erez O, et al. Maternal serum concentrations of the chemokine CXCL10/IP-10 are elevated in acute pyelonephritis during pregnancy. J Matern Fetal Neonatal Med. 2007;20(10):735–44.
Madan I, Than NG, Romero R, et al. The peripheral whole-blood transcriptome of acute pyelonephritis in human pregnancya. J Perinat Med. 2014;42(1):31–53.
Aluvihare VR, Kallikourdis M, Betz AG. Regulatory T cells mediate maternal tolerance to the fetus. Nat Immunol. 2004;5(3):266–71.
Lo YM, Lun FM, Chan KC, et al. Digital PCR for the molecular detection of fetal chromosomal aneuploidy. Proc Natl Acad Sci U S A. 2007;104(32):13116–21.
Fan HC, Blumenfeld YJ, Chitkara U, Hudgins L, Quake SR. Noninvasive diagnosis of fetal aneuploidy by shotgun sequencing DNA from maternal blood. Proc Natl Acad Sci U S A. 2008;105(42):16266–71.
Nicolaides KH, Syngelaki A, Ashoor G, Birdir C, Touzet G. Noninvasive prenatal testing for fetal trisomies in a routinely screened first-trimester population. Am J Obstet Gynecol. 2012;207(5):374 e371–6.
Song Y, Liu C, Qi H, Zhang Y, Bian X, Liu J. Noninvasive prenatal testing of fetal aneuploidies by massively parallel sequencing in a prospective Chinese population. Prenat Diagn. 2013;33(7):700–6.
Bianchi DW, Parker RL, Wentworth J, Madankumar R, Saffer C, Das AF, et al. DNA sequencing versus standard prenatal aneuploidy screening. N Engl J Med. 2014;370(9):799–808.
Comas C, Echevarria M, Rodriguez MA, Prats P, Rodriguez I, Serra B. Initial experience with non-invasive prenatal testing of cell-free DNA for major chromosomal anomalies in a clinical setting. J Matern Fetal Neonatal Med. 2015;28(10):1196–201.
Quezada MS, Gil MM, Francisco C, Orosz G, Nicolaides KH. Screening for trisomies 21, 18 and 13 by cell-free DNA analysis of maternal blood at 10-11 weeks' gestation and the combined test at 11-13 weeks. Ultrasound Obstet Gynecol. 2015;45(1):36–41.
Norton ME, Jacobsson B, Swamy GK, Laurent LC, Ranzini AC, Brar H, et al. Cell-free DNA analysis for noninvasive examination of trisomy. N Engl J Med. 2015;372(17):1589–97.
Petersen AK, Cheung SW, Smith JL, et al. Positive predictive value estimates for cell-free noninvasive prenatal screening from data of a large referral genetic diagnostic laboratory. Am J Obstet Gynecol. 2017;217(6):691 e691–6.
Iwarsson E, Jacobsson B, Dagerhamn J, Davidson T, Bernabe E, Heibert AM. Analysis of cell-free fetal DNA in maternal blood for detection of trisomy 21, 18 and 13 in a general pregnant population and in a high risk population - a systematic review and meta-analysis. Acta Obstet Gynecol Scand. 2017;96(1):7–18.
Society for Maternal-Fetal Medicine. Electronic address pso, Norton ME, Biggio JR, Kuller JA, Blackwell SC. The role of ultrasound in women who undergo cell-free DNA screening. Am J Obstet Gynecol. 2017;216(3):B2–7.
Liang B, Li H, He Q, Li H, Kong L, Xuan L, et al. Enrichment of the fetal fraction in non-invasive prenatal screening reduces maternal background interference. Sci Rep. 2018;8(1):17675.
Bianchi DW, Chiu RWK. Sequencing of circulating cell-free DNA during pregnancy. N Engl J Med. 2018;379(5):464–73.
Knight M, Redman CW, Linton EA, Sargent IL. Shedding of syncytiotrophoblast microvilli into the maternal circulation in pre-eclamptic pregnancies. Br J Obstet Gynaecol. 1998;105(6):632–40.
Redman CW, Sargent IL. Placental debris, oxidative stress and pre-eclampsia. Placenta. 2000;21(7):597–602.
Hahn S, Huppertz B, Holzgreve W. Fetal cells and cell free fetal nucleic acids in maternal blood: new tools to study abnormal placentation? Placenta. 2005;26(7):515–26.
Burton GJ, Jones CJ. Syncytial knots, sprouts, apoptosis, and trophoblast deportation from the human placenta. Taiwan J Obstet Gynecol. 2009;48(1):28–37.
Bianchi DW, Flint AF, Pizzimenti MF, Knoll JH, Latt SA. Isolation of fetal DNA from nucleated erythrocytes in maternal blood. Proc Natl Acad Sci U S A. 1990;87(9):3279–83.
Bianchi DW, Zickwolf GK, Yih MC, Flint AF, Geifman OH, Erikson MS, et al. Erythroid-specific antibodies enhance detection of fetal nucleated erythrocytes in maternal blood. Prenat Diagn. 1993;13(4):293–300.
Lo ES, Lo YM, Hjelm NM, Thilaganathan B. Transfer of nucleated maternal cells into fetal circulation during the second trimester of pregnancy. Br J Haematol. 1998;100(3):605–6.
Bianchi DW. Fetal cells in the mother: from genetic diagnosis to diseases associated with fetal cell microchimerism. Eur J Obstet Gynecol Reprod Biol. 2000;92(1):103–8.
Lo YM, Lau TK, Chan LY, Leung TN, Chang AM. Quantitative analysis of the bidirectional fetomaternal transfer of nucleated cells and plasma DNA. Clin Chem. 2000;46(9):1301–9.
Sekizawa A, Samura O, Zhen DK, Falco V, Farina A, Bianchi DW. Apoptosis in fetal nucleated erythrocytes circulating in maternal blood. Prenat Diagn. 2000;20(11):886–9.
Dunsmore G, Koleva P, Ghobakhloo N, et al. Lower abundance and impaired function of CD71+ erythroid cells in inflammatory bowel disease patients during pregnancy. J Crohns Colitis. 2019;13(2):230–244.
Livergood MC, LeChien KA, Trudell AS. Obesity and cell-free DNA "no calls": is there an optimal gestational age at time of sampling? Am J Obstet Gynecol. 2017;216(4):413 e411–9.
Rolnik DL, Yong Y, Lee TJ, Tse C, McLennan AC, da Silva Costa F. Influence of body mass index on fetal fraction increase with gestation and cell-free DNA test failure. Obstet Gynecol. 2018;132(2):436–43.
Mhatre M, Adeli S, Norwitz E, Craigo S, Phillippe M, Edlow A. The effect of maternal obesity on placental cell-free DNA release in a mouse model. Reprod Sci. 2019;26(9):1218–1224.
Mitsuya K, Parker AN, Liu L, Ruan J, Vissers MCM, Myatt L. Alterations in the placental methylome with maternal obesity and evidence for metabolic regulation. PLoS One. 2017;12(10):e0186115.
Higgins L, Mills TA, Greenwood SL, Cowley EJ, Sibley CP, Jones RL. Maternal obesity and its effect on placental cell turnover. J Matern Fetal Neonatal Med. 2013;26(8):783–8.
Polettini J, Behnia F, Taylor BD, Saade GR, Taylor RN, Menon R. Telomere fragment induced amnion cell senescence: a contributor to parturition? PLoS One. 2015;10(9):e0137188.
Sheller-Miller S, Urrabaz-Garza R, Saade G, Menon R. Damage-associated molecular pattern markers HMGB1 and cell-free fetal telomere fragments in oxidative-stressed amnion epithelial cell-derived exosomes. J Reprod Immunol. 2017;123:3–11.
Phillippe M, Phillippe SM. Birth and death: evidence for the same biologic clock. Am J Reprod Immunol. 2017;77(5).
Behnia F, Taylor BD, Woodson M, et al. Chorioamniotic membrane senescence: a signal for parturition? Am J Obstet Gynecol. 2015;213(3):359 e351–16.
Bonney EA, Krebs K, Saade G, et al. Differential senescence in feto-maternal tissues during mouse pregnancy. Placenta. 2016;43:26–34.
Menon R. Human fetal membranes at term: dead tissue or signalers of parturition? Placenta. 2016;44:1–5.
Menon R, Mesiano S, Taylor RN. Programmed fetal membrane senescence and exosome-mediated signaling: a mechanism associated with timing of human parturition. 2017;8:196–Front Endocrinol (Lausanne).
Sultana Z, Maiti K, Dedman L, Smith R. Is there a role for placental senescence in the genesis of obstetric complications and fetal growth restriction? Am J Obstet Gynecol. 2018;218(2S):S762–73.
Phillippe M, Adeli S. Cell-free DNA release by mouse placental explants. PLoS One. 2017;12(6):e0178845.
Sawyer MR, Adeli S, Phillippe M. Cell-free DNA release by mouse fetal membranes. Reprod Sci. 2018;1933719118817659.
Kacerovsky M, Celec P, Vlkova B, et al. Amniotic fluid protein profiles of intraamniotic inflammatory response to Ureaplasma spp. and other bacteria. PLoS One. 2013;8(3):e60399.
Romero R, Grivel JC, Tarca AL, et al. Evidence of perturbations of the cytokine network in preterm labor. Am J Obstet Gynecol. 2015;213(6):836 e831–18.
Nold C, Stone J, O'Hara K, et al. Block of granulocyte-macrophage colony-stimulating factor prevents inflammation-induced preterm birth in a mouse model for parturition. Reprod Sci. 2019;26(4):551–559.
Curry AE, Vogel I, Skogstrand K, Drews C, Schendel DE, Flanders WD, et al. Maternal plasma cytokines in early- and mid-gestation of normal human pregnancy and their association with maternal factors. J Reprod Immunol. 2008;77(2):152–60.
Ekelund CK, Vogel I, Skogstrand K, et al. Interleukin-18 and interleukin-12 in maternal serum and spontaneous preterm delivery. J Reprod Immunol. 2008;77(2):179–85.
Romero R, Chaemsaithong P, Docheva N, Korzeniewski SJ, Tarca AL, Bhatti G, et al. Clinical chorioamnionitis at term IV: the maternal plasma cytokine profile. J Perinat Med. 2016;44(1):77–98.
Tsou CL, Peters W, Si Y, et al. Critical roles for CCR2 and MCP-3 in monocyte mobilization from bone marrow and recruitment to inflammatory sites. J Clin Invest. 2007;117(4):902–9.
Akdis M, Aab A, Altunbulakli C, et al. Interleukins (from IL-1 to IL-38), interferons, transforming growth factor beta, and TNF-alpha: receptors, functions, and roles in diseases. J Allergy Clin Immunol. 2016;138(4):984–1010.
van Boeckel SR, Davidson DJ, Norman JE, Stock SJ. Cell-free fetal DNA and spontaneous preterm birth. Reproduction. 2018;155(3):R137–45.
Lin Y, Liu X, Shan B, Wu J, Sharma S, Sun Y. Prevention of CpG-induced pregnancy disruption by adoptive transfer of in vitro-induced regulatory T cells. PLoS One. 2014;9(4):e94702.
Jaiswal MK, Agrawal V, Mallers T, Gilman-Sachs A, Hirsch E, Beaman KD. Regulation of apoptosis and innate immune stimuli in inflammation-induced preterm labor. J Immunol. 2013;191(11):5702–13.
Fortunato SJ, Menon R, Lombardi SJ. Support for an infection-induced apoptotic pathway in human fetal membranes. Am J Obstet Gynecol. 2001;184(7):1392–7 discussion 1397-1398.
Menon R, Lombardi SJ, Fortunato SJ. TNF-alpha promotes caspase activation and apoptosis in human fetal membranes. J Assist Reprod Genet. 2002;19(4):201–4.
Gomez LM, Ma Y, Ho C, McGrath CM, Nelson DB, Parry S. Placental infection with human papillomavirus is associated with spontaneous preterm delivery. Hum Reprod. 2008;23(3):709–15.
Abrahams VM, Aldo PB, Murphy SP, et al. TLR6 modulates first trimester trophoblast responses to peptidoglycan. J Immunol. 2008;180(9):6035–43.
Mulla MJ, Myrtolli K, Tadesse S, et al. Cutting-edge report: TLR10 plays a role in mediating bacterial peptidoglycan-induced trophoblast apoptosis. Am J Reprod Immunol. 2013;69(5):449–53.
Lappas M. Cellular inhibitors of apoptosis proteins cIAP1 and cIAP2 are increased after labour in foetal membranes and myometrium and are essential for TNF-alpha-induced expression of pro-labour mediators. Am J Reprod Immunol. 2015;73(4):313–29.
Plazyo O, Romero R, Unkel R, Balancio A, Mial TN, Xu Y, et al. HMGB1 induces an inflammatory response in the chorioamniotic membranes that is partially mediated by the inflammasome. Biol Reprod. 2016;95(6):130.
Gomez-Lopez N, Romero R, Xu Y, et al. A role for the inflammasome in spontaneous labor at term with acute histologic chorioamnionitis. Reprod Sci. 2017;24(6):934–53.
Willcockson AR, Nandu T, Liu CL, Nallasamy S, Kraus WL, Mahendroo M. Transcriptome signature identifies distinct cervical pathways induced in lipopolysaccharide-mediated preterm birth. Biol Reprod. 2018;98(3):408–21.
Gotsch F, Romero R, Chaiworapongsa T, Erez O, Vaisbuch E, Espinoza J, et al. Evidence of the involvement of caspase-1 under physiologic and pathologic cellular stress during human pregnancy: a link between the inflammasome and parturition. J Matern Fetal Neonatal Med. 2008;21(9):605–16.
Gomez-Lopez N, Romero R, Xu Y, et al. Inflammasome assembly in the chorioamniotic membranes during spontaneous labor at term. Am J Reprod Immunol. 2017;77(5).
Romero R, Xu Y, Plazyo O, Chaemsaithong P, Chaiworapongsa T, Unkel R, et al. A role for the inflammasome in spontaneous labor at term. Am J Reprod Immunol. 2018;79(6):e12440.
Gomez-Lopez N, Romero R, Maymon E, et al. Clinical chorioamnionitis at term IX: in vivo evidence of intra-amniotic inflammasome activation. J Perinat Med. 2019;47(3):276–287.
Panaitescu B, Romero R, Gomez-Lopez N, et al. In vivo evidence of inflammasome activation during spontaneous labor at term. J Matern Fetal Neonatal Med. 2019;32(12):1978–1991.
Gomez-Lopez N, Romero R, Xu Y, Plazyo O, Unkel R, Leng Y, et al. A role for the inflammasome in spontaneous preterm labor with acute histologic chorioamnionitis. Reprod Sci. 2017;24(10):1382–401.
Modi BP, Teves ME, Pearson LN, Parikh HI, Haymond-Thornburg H, Tucker JL, et al. Mutations in fetal genes involved in innate immunity and host defense against microbes increase risk of preterm premature rupture of membranes (PPROM). Mol Genet Genomic Med. 2017;5(6):720–9.
Gomez-Lopez N, Romero R, Panaitescu B, Leng Y, Xu Y, Tarca AL, et al. Inflammasome activation during spontaneous preterm labor with intra-amniotic infection or sterile intra-amniotic inflammation. Am J Reprod Immunol. 2018;80(5):e13049.
Gomez-Lopez N, Motomura K, Miller D, Garcia-Flores V, Galaz J, Romero R. J Immunol. 2019 Dec 1;203(11):2757–2769. https://doi.org/10.4049/jimmunol.1900901.
Poltorak A, He X, Smirnova I, Liu MY, van Huffel C, du X, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282(5396):2085–8.
Hoshino K, Takeuchi O, Kawai T, et al. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J Immunol. 1999;162(7):3749–52.
Gomez-Lopez N, Romero R, Garcia-Flores V, et al. Inhibition of the NLRP3 inflammasome can prevent sterile intra-amniotic inflammation, preterm labor/birth and adverse neonatal outcomes. Biol Reprod. 2019;100(5):1306–1318.
Faro J, Romero R, Schwenkel G, et al. Intra-amniotic inflammation induces preterm birth by activating the NLRP3 inflammasome. Biol Reprod. 2019;100(5):1290–1305.
Acknowledgments
We thank the research assistants from the PRB Perinatal Translational Science Laboratory for their help with carrying out the Luminex assays, and Nicole Ducharme for her help with the DNA extraction and PCR assays. Finally, we thank Derek Miller, MSc, for his critical readings of the manuscript.
Funding
This research was supported, in part, by the Perinatology Research Branch (PRB), Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, US Department of Health and Human Services (NICHD/NIH/DHHS), and, in part, with federal funds from the NICHD/NIH/DHHS under Contract No. HHSN275201300006C. Dr. Roberto Romero has contributed to this work as part of his official duties as an employee of the United States Federal Government. This research was also supported by the Wayne State University Perinatal Initiative in Maternal, Perinatal, and Child Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict(s) of interest.
Electronic supplementary material
Supplementary Figure 1.
Plot showing the maternal plasma concentrations of IL-10 at 12.5 – 19.5 days post coitum (dpc), in labor, and 12 – 36 h postpartum. Each dot represents one maternal plasma sample. Midlines = medians, boxes = interquartile range, whiskers = minimum and maximum range. Red curves indicate trends over time. N = 5 – 11 dams per group. (PNG 62 kb)
Supplementary Figure 2.
Plots showing the negative correlations between plasma concentrations of IL-6 or IL-4 and the number of GFP-positive pups prior to systemic inflammation-induced preterm birth. Spearman correlation coefficients and p-values are displayed. Asterisk (*) indicates p-value obtained using a one-tailed Mann-Whitney U test. N = 8 dams. (PNG 83 kb)
Supplementary Figure 3.
Plot showing a negative correlation between plasma concentrations of IL-4 and cell-free fetal DNA (Egfp) levels prior to systemic inflammation-induced preterm birth. Spearman correlation coefficients and p-values are displayed. N = 8 dams. (PNG 80 kb)
ESM 1
(DOC 50 kb)
Rights and permissions
About this article
Cite this article
Gomez-Lopez, N., Romero, R., Schwenkel, G. et al. Cell-Free Fetal DNA Increases Prior to Labor at Term and in a Subset of Preterm Births. Reprod. Sci. 27, 218–232 (2020). https://doi.org/10.1007/s43032-019-00023-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-019-00023-6