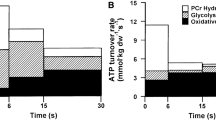
Abstract
Background
Skeletal muscle glycogen is an important energy source for muscle contraction and a key regulator of metabolic responses to exercise. Manipulation of muscle glycogen is therefore a strategy to improve performance in competitions and potentially adaptation to training. However, assessing muscle glycogen in the field is impractical, and there are no normative values for glycogen concentration at rest and during exercise.
Objective
The objective of this study was to meta-analyse the effects of fitness, acute dietary carbohydrate (CHO) availability and other factors on muscle glycogen concentration at rest and during exercise of different durations and intensities.
Data Source and Study Selection
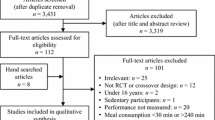
PubMed was used to search for original articles in English published up until February 2018. Search terms included muscle glycogen and exercise, filtered for humans. The analysis incorporated 181 studies of continuous or intermittent cycling and running by healthy participants, with muscle glycogen at rest and during exercise determined by biochemical analysis of biopsies.
Data Analysis
Resting muscle glycogen was determined with a meta-regression mixed model that included fixed effects for fitness status [linear, as maximal oxygen uptake (\(\dot{V}\)O2max) in mL·kg−1·min−1] and CHO availability (three levels: high, ≥ 6 g·kg−1 of CHO per day for ≥ 3 days or ≥ 7 g·kg−1 CHO per day for ≥ 2 days; low, glycogen depletion and low-CHO diet; and normal, neither high nor low, or not specified in study). Muscle glycogen during exercise was determined with a meta-regression mixed model that included fixed effects for fitness status, resting glycogen [linear, in mmol·kg−1 of dry mass (DM)], exercise duration (five levels, with means of 5, 23, 53 and 116 min, and time to fatigue), and exercise intensity (linear, as percentage of \(\dot{V}\)O2max); intensity, fitness and resting glycogen were interacted with duration, and there were also fixed effects for exercise modes, CHO ingestion, sex and muscle type. Random effects in both models accounted for between-study variance and within-study repeated measurement. Inferences about differences and changes in glycogen were based on acceptable uncertainty in standardised magnitudes, with thresholds for small, moderate, large and very large of 25, 75, 150 and 250 mmol·kg−1 of DM, respectively.
Results
The resting glycogen concentration in the vastus lateralis of males with normal CHO availability and \(\dot{V}\)O2max (mean ± standard deviation, 53 ± 8 mL·kg−1·min−1) was 462 ± 132 mmol·kg−1. High CHO availability was associated with a moderate increase in resting glycogen (102, ± 47 mmol·kg−1; mean ± 90% confidence limits), whereas low availability was associated with a very large decrease (− 253, ± 30 mmol·kg−1). An increase in \(\dot{V}\)O2max of 10 mL·kg−1·min−1 had small effects with low and normal CHO availability (29, ± 44 and 67, ± 15 mmol·kg-1, respectively) and a moderate effect with high CHO availability (80, ± 40 mmol·kg−1). There were small clear increases in females and the gastrocnemius muscle. Clear modifying effects on glycogen utilisation during exercise were as follows: a 30% \(\dot{V}\)O2max increase in intensity, small (41, ± 20 mmol·kg−1) at 5 min and moderate (87–134 mmol·kg−1) at all other timepoints; an increase in baseline glycogen of 200 mmol·kg−1, small at 5–23 min (28–59 mmol·kg−1), moderate at 116 min (104, ± 15 mmol·kg−1) and moderate at fatigue (143, ± 33 mmol·kg−1); an increase in \(\dot{V}\)O2max of 10 mL·kg−1·min−1, mainly clear trivial effects; exercise mode (intermittent vs. continuous) and CHO ingestion, clear trivial effects. Small decreases in utilisation were observed in females (vs. males: − 30, ± 29 mmol·kg−1), gastrocnemius muscle (vs. vastus lateralis: − 31, ± 46 mmol·kg−1) and running (vs. cycling: − 70, ± 32 mmol·kg−1).
Conclusion
Dietary CHO availability and fitness are important factors for resting muscle glycogen. Exercise intensity and baseline muscle glycogen are important factors determining glycogen use during exercise, especially with longer exercise duration. The meta-analysed effects may be useful normative values for prescription of endurance exercise.






Similar content being viewed by others
References
Bergström J. Muscle electrolytes in man. Determined by neutron activation analysis on needle biopsy specimens. Scand J Clin Lab Investig Engl. 1962;14(Suppl 68).
Bergström J, Hultman E. Muscle glycogen synthesis after exercise: an enhancing factor localized to the muscle cells in man. Nature. 1966;210:309–10.
Bergström J, Hultman E. The effect of exercise on muscle glycogen and electrolytes in normals. Scand J Clin Lab Invest. 1966;18:16–20.
Bergström J, Hultman E. A study of the glycogen metabolism during exercise in man. Scand J Clin Lab Invest. 1967;19:218–28.
Bergström J, Hermansen L, Hultman E, Saltin B. Diet, muscle glycogen and physical performance. Acta Physiol. 1967;71:140–50.
Karlsson J, Saltin B. Diet, muscle glycogen, and endurance performance. J Appl Physiol. 1971;31:203–6.
American Dietetic Association. Position of the American Dietetic Association and the Canadian Dietetic Association: nutrition for physical fitness and athletic performance for adults. J Am Diet Assoc. 1993;93:691–6.
Hansen AK. Skeletal muscle adaptation: training twice every second day vs. training once daily. J Appl Physiol. 2004;98:93–9.
Marquet L-A, Brisswalter J, Louis J, Tiollier E, Burke LM, Hawley JA, et al. Enhanced endurance performance by periodization of carbohydrate intake: sleep low strategy. Med Sci Sports Exerc. 2016;48:663–72.
Bartlett JD, Hawley JA, Morton JP. Carbohydrate availability and exercise training adaptation: too much of a good thing? Eur J Sport Sci. 2015;15:3–12.
Hawley JA, Morton JP. Ramping up the signal: promoting endurance training adaptation in skeletal muscle by nutritional manipulation. Clin Exp Pharmacol Physiol. 2014;41:608–13.
Lane SC, Camera DM, Lassiter DG, Areta JL, Bird SR, Yeo WK, et al. Effects of sleeping with reduced carbohydrate availability on acute training responses. J Appl Physiol. 2015;119:643–55.
Philp A, Hargreaves M, Baar K. More than a store: regulatory roles for glycogen in skeletal muscle adaptation to exercise. AJP Endocrinol Metab. 2012;302:E1343–51.
Impey SG, Hearris MA, Hammond KM, Bartlett JD, Louis J, Close GL, et al. Fuel for the work required: a theoretical framework for carbohydrate periodization and the glycogen threshold hypothesis. Sports Med. 2018;48:1031–48.
Greene J, Louis J, Korostynska O, Mason A. State-of-the-art methods for skeletal muscle glycogen analysis in athletes—the need for novel non-invasive techniques. Biosensors. 2017;7:11.
Coyle EF, Coggan AR, Hemmert MK, Ivy JL. Muscle glycogen utilization during prolonged strenuous exercise when fed carbohydrate. J Appl Physiol. 1986;61:165–72.
Hermansen L, Hultman E, Saltin B. Muscle glycogen during prolonged severe exercise. Acta Physiol. 1967;71:129–39.
Gollnick PD, Piehl K, Saltin B. Selective glycogen depletion pattern in human muscle fibres after exercise of varying intensity and at varying pedalling rates. J Physiol. 1974;241:45.
van Loon LJ, Greenhaff PL, Constantin-Teodosiu D, Saris WH, Wagenmakers AJ. The effects of increasing exercise intensity on muscle fuel utilisation in humans. J Physiol. 2001;536:295–304.
Romijn JA, Coyle EF, Sidossis LS, Gastaldelli A, Horowitz JF, Endert E, et al. Regulation of endogenous fat and carbohydrate metabolism in relation to exercise intensity and duration. Am J Physiol. 1993;265:E380–91.
Galbo H, Holst JJ, Christensen NJ. The effect of different diets and of insulin on the hormonal response to prolonged exercise. Acta Physiol Scand. 1979;107:19–32.
Hargreaves M, McConell G, Proietto J. Influence of muscle glycogen on glycogenolysis and glucose uptake during exercise in humans. J Appl Physiol. 1995;78:288–92.
Blomstrand E, Saltin B. Effect of muscle glycogen on glucose, lactate and amino acid metabolism during exercise and recovery in human subjects. J Physiol. 1999;514:293–302.
Karlsson J, Nordesjö L-O, Saltin B. Muscle glycogen utilization during exercise after physical training. Acta Physiol Scand. 1974;90:210–7.
Young AJ, Evans WJ, Cymerman A, Pandolf KB, Knapik JJ, Maher JT. Sparing effect of chronic high-altitude exposure on muscle glycogen utilization. J Appl Physiol. 1982;52:857–62.
Yang M. A review of random effects modelling in SAS (release 8.2). London: Centre for Multilevel Modelling; 2003.
Hopkins WG, Marshall SW, Batterham AM, Hanin J. Progressive statistics for studies in sports medicine and exercise science. Med Sci Sports Exerc. 2009;41:3–13.
Burke LM, van Loon LJC, Hawley JA. Postexercise muscle glycogen resynthesis in humans. J Appl Physiol. 2017;122:1055–67.
Coyle EF, Jeukendrup AE, Oseto MC, Hodgkinson BJ, Zderic TW. Low-fat diet alters intramuscular substrates and reduces lipolysis and fat oxidation during exercise. Am J Physiol Endocrinol Metab. 2001;280:E391–8.
Sherman WM, Costill DL, Fink WJ, Miller JM. Effect of exercise-diet manipulation on muscle glycogen and its subsequent utilization during performance. Int J Sports Med. 1981;2:114–8.
Burke LM, Hawley JA, Schabort EJ, St Clair Gibson A, Mujika I, Noakes TD. Carbohydrate loading failed to improve 100-km cycling performance in a placebo-controlled trial. J Appl Physiol. 1985;2000(88):1284–90.
Rauch LH, Rodger I, Wilson GR, Belonje JD, Dennis SC, Noakes TD, et al. The effects of carbohydrate loading on muscle glycogen content and cycling performance. Int J Sport Nutr. 1995;5:25–36.
Green HJ, Burnett M, Jacobs I, Ranney D, Smith I, Tupling S. Adaptations in muscle metabolic regulation require only a small dose of aerobic-based exercise. Eur J Appl Physiol. 2013;113:313–24.
Howarth KR, Burgomaster KA, Phillips SM, Gibala MJ. Exercise training increases branched-chain oxoacid dehydrogenase kinase content in human skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1335–41.
Roedde S, MacDougall JD, Sutton JR, Green HJ. Supercompensation of muscle glycogen in trained and untrained subjects. Can J Appl Sport Sci. 1986;11:42–6.
Sherman WM, Doyle JA, Lamb DR, Strauss RH. Dietary carbohydrate, muscle glycogen, and exercise performance during 7 d of training. Am J Clin Nutr. 1993;57:27–31.
Mcinerney P, Lessard SJ, Burke LM, Coffey VG, Lo Giudice SL, Southgate RJ, et al. Failure to repeatedly supercompensate muscle glycogen stores in highly trained men. Med Sci Sports Exerc. 2005;37:404–11.
Blanchard MA, Jordan G, Desbrow B, MacKinnon LT, Jenkins DG. The influence of diet and exercise on muscle and plasma glutamine concentrations. Med Sci Sports Exerc. 2001;33:69–74.
Holloszy JO. Biochemical adaptations to exercise: aerobic metabolism. Exerc Sport Sci Rev. 1973;1:45–71.
Holloszy JO, Coyle EF. Adaptations of skeletal muscle to endurance exercise and their metabolic consequences. J Appl Physiol. 1984;56:831–8.
Coggan AR, Kohrt WM, Spina RJ, Kirwan JP, Bier DM, Holloszy JO. Plasma glucose kinetics during exercise in subjects with high and low lactate thresholds. J Appl Physiol. 1992;73:1873–80.
Coyle EF, Coggan AR, Hopper MK, Walters TJ. Determinants of endurance in well-trained cyclists. J Appl Physiol Bethesda Md. 1985;1988(64):2622–30.
Faude O, Kindermann W, Meyer T. Lactate threshold concepts. Sports Med. 2009;39:469–90.
Hickson RC, Bomze HA, Holloszy JO. Linear increase in aerobic power induced by a strenuous program of endurance exercise. J Appl Physiol. 1977;42:372–6.
Scribbans TD, Vecsey S, Hankinson PB, Foster WS, Gurd BJ. The effect of training intensity on VO2max in young healthy adults: a meta-regression and meta-analysis. Int J Exerc Sci. 2016;9:230.
Wang L, Psilander N, Tonkonogi M, Ding S, Sahlin K. Similar expression of oxidative genes after interval and continuous exercise. Med Sci Sports Exerc. 2009;41:2136–44.
Bartlett JD, Hwa Joo C, Jeong TS, Louhelainen J, Cochran AJ, Gibala MJ, et al. Matched work high-intensity interval and continuous running induce similar increases in PGC-1alpha mRNA, AMPK, p38, and p53 phosphorylation in human skeletal muscle. J Appl Physiol. 2012;112:1135–43.
Palmer GS, Borghouts LB, Noakes TD, Hawley JA. Metabolic and performance responses to constant-load vs. variable-intensity exercise in trained cyclists. J Appl Physiol. 1999;87:1186–96.
Fisher DJ, Carpenter JR, Morris TP, Freeman SC, Tierney JF. Meta-analytical methods to identify who benefits most from treatments: daft, deluded, or deft approach? BMJ. 2017;356:j573.
Hopkins WG. Improving meta-analyses in sport and exercise science. Sportscience. 2018;22:11–7.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No sources of funding were used to assist in the preparation of this article.
Conflict of Interest
José L. Areta and Will G. Hopkins declare that they have no conflicts of interest relevant to the content of this review.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Areta, J.L., Hopkins, W.G. Skeletal Muscle Glycogen Content at Rest and During Endurance Exercise in Humans: A Meta-Analysis. Sports Med 48, 2091–2102 (2018). https://doi.org/10.1007/s40279-018-0941-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40279-018-0941-1