Abstract
Objectives
To compare anxiolysis produced by intranasal clonidine with intranasal midazolam as premedication in children undergoing surgery.
Design
Double-blind randomized controlled study.
Setting
Tertiary-care hospital, July 2009 to June 2010.
Patients
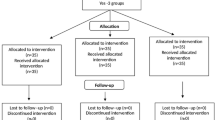
60 American Society of Anesthesiologists physical status I–II surgical patients aged 1–10 yr.
Intervention
Participants randomly allocated to receive either intranasal clonidine 4 mcg/kg (Group I) with atropine or intranasal midazolam 0.3 mg/kg (Group II).
Outcome Measures
Primary: satisfactory anxiolysis at 30 min after drug administration. Secondary: satisfactory mask acceptance, times of onset of sedation and anxiolysis, drug acceptance, level of sedation, wake-up score and side effects.
Results
All children achieved satisfactory anxiolysis at 30 min. Group I fared significantly better than Group-II on mask acceptance (100% in Group I vs. 80% in Group II; P=0.024), drug acceptance (93% vs. 13%; P<0.001) and proportion of patients with satisfactory wake-up scores (100% vs. 53%; P<0.001). Group II patients had significantly faster onset of sedation (median 10 min vs. 15 min; P<0.05) but not that of anxiolysis compared to Group-I (median 10 min for both groups; P>0.05). Side effects were significantly more frequent in Group II.
Conclusions
Though intranasal midazolam produced faster sedation, both the drugs produced satisfactory anxiolysis at 30 min.
Similar content being viewed by others
References
McCann ME, Kain ZN. The management of preoperative anxiety in children. Anesth Analg. 2001;93:98–105.
Kain Z, Mayes L. Anxiety in children during the perioperative period. In: Borestein M, Genevro J, Mahwah NJ, eds. Child Development and Behavioral Pediatrics. Mahwah: Lawrence Erlbaum Associates, 1996. p. 85–103.
Kain Z, Mayes L, Wang S, Caramico LA, Hofstadter MB. Parental presence during induction of anesthesia vs. sedative premedication: which intervention is more effective? Anesthesiology. 1998;89:1147–1156.
Kain ZN, Magnus LC, Bell C, Weisman S, Hofstadter MB, Rimar S. Premedication in United States — a status report. Anesth Analg. 1997;84:427–432.
Bergendahl H, Lonnqvist PA, Eksborg S. Clonidine in paediatric anaesthesia: review of literature and comparison with benzodiazepines for premedication. Acta Anaesthesiol Scand. 2006;50:135–143.
Lonnqvist PA, Habre W. Midazolam as premedication: is the Emperor naked or just half-dressed? Pediatr Anesth. 2005;15:263–265.
Basker S, Singh G, Jacob R. Clonidine in paediatrics — a review. Indian J Anaesth. 2009;53:270–280.
Dahmani S, Brasher C, Stany I, Golmard J, Skhiri A, Bruneau B, et al. Premedication with clonidine is superior to benzodiazepines. A meta-analysis of published studies. Acta Anaesthesiol Scand. 2010;54:397–402.
Almenrader N, Passariello M, Coccetti B, Haiberger R, Pietropaoli P. Steal induction after clonidine premedication: Comparison of oral and nasal route. Pediatr Anesth. 2007;17:230–234.
Stella MJ, Bailey AG. Intranasal clonidine as a premedicant: three cases with unique indications. Pediatr Anesth. 2008;18:71–73.
Almenrader N, Larsson P, Passariello M, Haiberger R, Pietropaoli P, Lönnqvist PA, et al. Absorption pharmacokinetics of clonidine nasal drops in children. Pediatr Anesth. 2009;19:257–261.
Bergendahl HT, Lönnqvist PA, Eksborg S, Ruthström E, Nordenberg L, Zetterqvist H, et al. Clonidine vs. midazolam as premedication in children undergoing adenotonsillectomy: a prospective, randomized, controlled clinical trial. Acta Anaesthesiol Scand. 2004;48:1292–1300.
Almenrader N, Passariello M, Coccetti B, Haiberger R, Pietropaoli P, et al. Premedication in children: a comparison of oral midazolam and oral clonidine. Pediatr Anesth. 2007;17:1143–1149.
Ramsay MA, Savege TM, Simpson BR, Goodwin R. Controlled sedation with alphaxalone-alphadolone. BMJ. 1974;2:656–659.
Kogan A, Katz J, Efrat R, Eidelman LA. Premedication with midazolam in young children: a comparison of 4 routes of administration. Pediatr Anesth. 2002;12:685–689.
Mukherjee S, Ray M, Ray A, Khanra M, Mandal PK, Pal R. Clonidine premedication for paediatric patients: a comparison of the oral and nasal route. J Anaesth Clin Pharmacol. 2010;26:319–322.
Yuen VM, Hui TW, Irwin MG, Yuen MK. A comparison of intranasal dexmedetomidine and oral midazolam for premedication in pediatric anesthesia: a double blind randomized controlled trial. Anesth Analg. 2008;106:1715–1721.
Karl HW, Rosenberger JL, Larach MG, Ruffle JM. Transmucosal administration of midazolam for premedication of patients — comparison of nasal and sublingual route. Anesthesiology. 1993;78:885–891.
Bhakta P, Ghosh BR, Roy M, Mukherjee G. Evaluation of intranasal midazolam for preanaesthetic sedation in paediatric patients. Indian J Anaesth. 2007;51:111–116.
Georgitis JW. Nasal atropine sulfate: efficacy and safety of 0.050% and 0.075% solutions for severe rhinorrhea. Arch Otolaryngol Head Neck Surg. 1998;124:916–920.
Takeuchi K, Suzumura E, Majima Y, Sakakura Y. Effect of atropine on nasal mucociliary clearance. Acta Otolaryngol. 1990;110:120–123.
Griffith N, Howell S, Mason DG. Intranasal midazolam for premedication of children undergoing day-case anaesthesia: comparison of two delivery systems with assessment of intra-observer variability. Br J Anaesth. 1998;81:865–869.
Malinvosky JM, Populare C. Premedication with midazolam in children: effects of intranasal, rectal and oral route on plasma midazolam concentration. Anaesthesia. 1995;50:351–354.
Larsson P, Eksborg S, Lönnqvist PA. Onset time for pharmacologic premedication with clonidine as a nasal aerosol: a double-blind, placebo-controlled, randomized trial. Ped Anesth. 2012;22:877–883.
Kain ZN, Mayes LC, Cicchetti DV, Bagnall AL, Finley JD, Hofstadter MB. The Yale Preoperative Anxiety Scale: how does it compare with a “gold standard”? Anesth Analg. 1997;85:783–788.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mitra, S., Kazal, S. & Anand, L.K. Intranasal Clonidine vs. Midazolam as Premedication in Children: A Randomized Controlled Trial . Indian Pediatr 51, 113–118 (2014). https://doi.org/10.1007/s13312-014-0352-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13312-014-0352-9