Abstract
Purpose
Intranasal dexmedetomidine premedication is a newly introduced method for reducing stress and anxiety before general anesthesia in children. We performed a meta-analysis to identify the effects of intranasal dexmedetomidine premedication in children.
Source
We conducted a systematic review to find published randomized-controlled trials using intranasal dexmedetomidine as premedication. We searched databases in EMBASE™, MEDLINE®, and the Cochrane Controlled Trials Register using the Ovid platform. This study was conducted based on the Cochrane Review Methods.
Principal findings
This review included 1,168 participants in 13 studies. Intranasal dexmedetomidine premedication provided more satisfactory sedation at parent separation (relative risk [RR], 1.45; 95% confidence interval [CI], 1.19 to 1.76; P = 0.0002; I2 = 80%) than other premedication regimes. In addition, it reduced the need for rescue analgesics (RR, 0.58; 95% CI, 0.40 to 0.83; P = 0.003; I2 = 0%). Nevertheless, there were no differences in sedation at mask induction (RR, 1.25; 95% CI, 0.98 to 1.59; P = 0.08; I2 =71%) or in the incidence of emergence delirium (RR, 0.52; 95% CI, 0.24 to 1.13; P = 0.10; I2 = 67%). Intranasal dexmedetomidine was associated with a significantly lower incidence of nasal irritation (RR, 0.05; 95% CI, 0.01 to 0.36; P = 0.003; I2 = 0%) and postoperative nausea and vomiting (RR, 0.63; 95% CI, 0.40 to 0.99; P = 0.04; I2 = 0%) than other premedication treatments. It also showed significantly lower systolic blood pressure (weighted mean difference [WMD], −6.7 mmHg; 95% CI, −10.5 to −2.9; P = 0.0006; I2 = 96%) and heart rate (WMD, −6.8 beats·min−1; 95% CI, −11.3 to −2.6; P = 0.002; I2 = 98%).
Conclusions
Intranasal dexmedetomidine provided more satisfactory sedation at parent separation and reduced the need for rescue analgesics and the incidence of nasal irritation and postoperative nausea and vomiting when compared with other premedication treatments.
Résumé
Objectif
La dexmédétomidine intranasale est une prémédication nouvellement introduite qui permet de réduire le stress et l’anxiété avant une anesthésie générale chez l’enfant. Nous avons réalisé une méta-analyse afin d’identifier les effets de la prémédication intranasale de dexmédétomidine chez l’enfant.
Source
Nous avons entrepris une revue systématique de la littérature afin d’extraire les études randomisées contrôlées publiées qui avaient examiné l’administration intranasale de dexmédétomidine en prémédication. À l’aide de la plateforme Ovid, nous avons effectué des recherches dans les bases de données EMBASE™, MEDLINE® et dans le Registre des études contrôlées Cochrane. Cette étude a été réalisée selon la méthodologie de révision Cochrane.
Constatations principales
Ce compte-rendu a inclus 1168 participants tirés de 13 études. La prémédication intranasale de dexmédétomidine a procuré une sédation plus satisfaisante lors du moment de séparation d’avec les parents (risque relatif [RR], 1,45; intervalle de confiance [IC] 95 %, 1,19 à 1,76; P = 0,0002; I2 = 80 %) que les autres régimes de prémédication. En outre, ce régime posologique a réduit le besoin en analgésiques de sauvetage (RR, 0,58; IC 95 %, 0,40 à 0,83; P = 0,003; I2 = 0 %). Toutefois, aucune différence n’a été observée au niveau de la sédation au moment de l’induction au masque (RR, 1,25; IC 95 %, 0,98 à 1,59; P = 0,08; I2 =71 %) ou dans l’incidence de délirium au réveil (RR, 0,52; IC 95 %, 0,24 à 1,13; P = 0,10; I2 =67 %). La dexmédétomidine intranasale a été associée à une incidence significativement plus basse d’irritation nasale (RR, 0,05; IC 95 %, 0,01 à 0,36; P = 0,003; I2 = 0 %) et de nausées et vomissements postopératoires (RR, 0,63; IC 95 %, 0,40 à 0,99; P = 0,04; I2 = 0 %) que les autres traitements en prémédication. Une baisse significative de la tension artérielle systolique (différence moyenne pondérée [DMP], −6,67 mmHg; IC 95 %, −10,50 à −2,85; P = 0,0006; I2 = 96 %) ainsi que de la fréquence cardiaque (DMP, −6,81 battements·min−1; IC 95 %, −11,03 à −2,59; P = 0,002; I2 = 98 %) a également été observée.
Conclusion
Par rapport aux autres traitements en prémédication, la dexmédétomidine intranasale a procuré une sédation plus satisfaisante lors de la séparation de l’enfant et du parent et réduit le besoin d’analgésiques de sauvetage, l’incidence d’irritation nasale ainsi que les nausées et vomissements postopératoires.
Similar content being viewed by others
Premedication in children is helpful for both separating the child from their parent and reducing the child’s stress and anxiety, thus facilitating smooth induction of anesthesia. Even though intended procedures are explained to children in appropriate detail, they are anxious about needle sticks and are often technically challenging to sedate. Furthermore, the drugs given for this purpose should have little effect on hemodynamics and respiration so as to allow the child to recover quickly and to facilitate early discharge without side effects. Several approaches have been attempted to achieve this goal.1
To sedate a child, clinicians commonly use intravenous drug administration. Nevertheless, since intravenous cannulation is painful and often requires the use of restraints, it could lead to long-term psychological problems in the child, such as refusing contact with healthcare professionals.2 Therefore, various routes for premedication have been used to alleviate the pain of intravenous cannulation. Intranasal premedication does not require venous puncture and represents a potential alternative administrative route for children. This site has rich vascularization and good drug permeability; hence, intranasal administration leads to rapid absorption into systemic circulation and ensuing effective and rapid sedation.3,4 Dexmedetomidine is a potent, highly selective, and specific alpha-2 adrenoreceptor agonist with both sedative and analgesic effects.5,6 When dexmedetomidine is administered through the nasal mucosa, it is an easy and noninvasive route with a high bioavailability of 81.8%.7 Until now, the relative effectiveness of intranasal dexmedetomidine compared with other intranasal or oral premedicants remains incompletely studied. Therefore, we conducted this study to identify the efficacy and safety of premedication with intranasal dexmedetomidine in children. We performed a meta-analysis of randomized-controlled trials comparing intranasal dexmedetomidine with other intranasal or oral premedications.
Methods
We used a systematic approach to identify publications that evaluated the efficacy and safety of intranasal dexmedetomidine premedication in children. This systematic review and meta-analysis is based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) and the Cochrane Review Methods.8
Data sources and literature sources
We searched EMBASE™ (from 1974), MEDLINE® In-Process & Other Non-Indexed Citations, Ovid MEDLINE (R) Daily, Ovid MEDLINE (R) 1946 to present, Cochrane Controlled Trials Register, and Cochrane Database of Systematic Reviews. We used the OVID platform to examine each source from its inauguration to November 3, 2016. In addition, we performed a literature search of Web of Science®, Google Scholar, and KoreaMed databases to retrieve the relevant studies. The main keywords were dexmedetomidine, intranasal drug administration, and randomized-controlled trial.
Study selection
Two reviewers (J.Y.K. and J.H.J.) independently identified all the studies using predefined selection criteria. A third reviewer (K.N.K.) arbitrated disagreements that occurred in the primary study selection. Studies were included in this meta-analysis if they satisfied the following criteria: 1) Literature type: randomized-controlled trials in all published international journals without language restriction; 2) Subjects: children undergoing premedication treatment before surgery; 3) Interventions: studies evaluating the efficacy and safety of intranasal dexmedetomidine premedication; 4) Outcomes: the primary outcomes were sedation at separation from patients, sedation at anesthesia mask induction, and the incidence of emergence agitation; secondary outcomes were the need for postoperative rescue analgesia, duration of stay in the postanesthesia care unit, hemodynamic changes, and adverse effects (e.g., incidence of nausea and vomiting, nasal irritation, laryngospasm, and shivering). The outcome variables are the incidence of events or mean differences between groups.
Data extraction
Two reviewers (J.Y.K. and J.H.J.) independently abstracted the data using a pre-specified data abstraction form. The third reviewer (K.N.K.) then verified the abstracted data. The following variables were abstracted: 1) the number of patients and patient characteristics; 2) the protocol for premedication administration method and dose; 3) the incidence of events or means and standard deviations of the outcome data; 4) the time point of outcome data measurement; and 5) the incidence of adverse events in each method. If the variables were not reported in an article, we emailed the authors to request the data.
Assessment of methodological quality
Two reviewers (K.N.K. and J.H.J.) independently assessed the risk of bias using the Cochrane risk of bias tool, which considers the methods of random sequence generation, allocation concealment, blinding of participants and the outcome estimator, incomplete reporting of outcome data, selective reporting of outcomes, and other sources of bias risk.
Quality of the evidence
We used the GRADE (Grades of Recommendation, Assessment, Development and Evaluation) Working Group system to evaluate the quality of the evidence.8 Two reviewers (K.N.K. and J.H.J.) independently assessed the quality of each outcome. The five categories used for the GRADE quality assessment were: limitations of design, inconsistency, indirectness, imprecision, and publication bias. We used GRADE profiler (GRADEpro) software to create the “Summary of findings” table (Table 3), which includes the following outcomes: 1) satisfactory sedation at parent separation; 2) satisfactory sedation at mask induction; 3) incidence of emergency agitation; 4) requirement of rescue analgesics; 5) incidence of nasal irritation; 6) systolic blood pressure (SBP); and 7) heart rate.
Statistical analysis
We report continuous data as mean differences and their associated 95% confidence intervals (CIs) with analyses using weighted mean differences (WMDs) determined via the generic inverse variance method. Binary outcomes are reported as a risk ratio (RR) with 95% CI. Heterogeneity between studies was assessed using the χ2 test and the I2 statistic.9 We considered an I2 statistic > 50% and a χ2 test with a P value < 0.10 to indicate statistical heterogeneity. We used random effects models when significant statistical or clinical heterogeneity was detected.
Subgroup analysis was performed according to the premedication regimes to evaluate the effect of each premedication method. To evaluate how the risk of bias could affect our estimates, we conducted sensitivity analysis by analyzing only studies with a low risk of bias. The studies with more than one area of unclear or high risk of bias were excluded from analysis. We used funnel plots to assess publication bias of the studies included in this meta-analysis. All statistical analyses were conducted using the Cochrane Collaboration Review Manager Software (RevMan version 5.2).
Results
Identification of studies
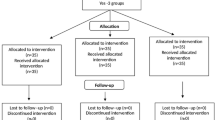
Initial database searches identified 273 publications. After removing 144 duplicated articles, we further excluded 106 articles by screening their titles and abstracts. Following review of the full manuscripts for the remaining 23 publications, we identified 13 publications reporting potentially relevant studies. The other ten articles were eliminated due to different study designs (four articles), only a reported abstract (one article), and inappropriate outcome data (five articles). Consequently, we included 13 studies10,11,12,13,14,15,16,17,18,19,20,21,22 and 1,168 participants in this meta-analysis (Fig. 1).
Study characteristics and patient populations
The included articles were undertaken from 2008-2016 in eight different countries: USA (one), Turkey (one), Saudi Arabia (one), India (three), China (three), Egypt (two), Mexico (one), and Oman (one). Four studies13,18,19,22 compared the effects of intranasal dexmedetomidine with those of oral midazolam, and six studies10,11,14,16,17,21 compared the effects of intranasal dexmedetomidine with those of intranasal midazolam. One study compared intranasal dexmedetomidine with intranasal clonidine,15 and two studies compared intranasal dexmedetomidine with intranasal normal saline.12,20 One study additionally compared intranasal dexmedetomidine with intranasal ketamine,14 and one study additionally compared intranasal dexmedetomidine with intranasal normal saline.10 The characteristics of the included studies are summarized in Table 1.
Quality of the included studies
All of the included studies used a random allocation method. Ten studies10,11,12,13,14,15,16,17,18,22 described the allocation concealment in detail, and six studies10,11,13,15,16,22 concretely explained their blinding methods. The risk of allocation concealment and blinding was unclear in the other studies. In most studies, there was low risk of incomplete outcome data and selective reporting. Risk of bias graphs and summaries are presented in Fig. 2A and B.
Publication bias
Funnel plots of the outcomes did not show a symmetrical shape (Electronic Supplementary Material Figs 1-3); however, the accuracy of the funnel plots is uncertain due to the low (i.e., < 10) number of included studies.8
Satisfactory sedation at parent separation
Satisfactory sedation at parent separation was reported in nine randomized trials11,13,14,16,17,18,19,21,22 with 896 patients. Satisfactory sedation at parent separation was evaluated by sedation scores on a four-point sedation scale14,16,17,18 and on the Modified Observer’s Assessment of Alertness/Sedation Scale.11,19,21,22 Each study determined a sleepy or lethargic response to parent separation as a satisfactory level of sedation. We found that patients who were premedicated with intranasal dexmedetomidine were significantly sedated at parent separation when compared with other premedication treatments (RR, 1.45; 95% CI, 1.19 to 1.76; P = 0.0002; I2 = 80%) (Fig. 3A). A subgroup analysis of the trials comparing intranasal dexmedetomidine with oral midazolam revealed that intranasal dexmedetomidine was more effective than oral midazolam (RR, 1.56; 95% CI, 1.15 to 2.11; P = 0.005; I2 = 82%). There was no difference between intranasal dexmedetomidine and intranasal midazolam (RR, 1.42; 95% CI, 0.96 to 2.11; P = 0.08; I2 = 85%).
Satisfactory sedation at mask induction
Seven trials11,13,16,17,18,19,21 with 648 patients compared satisfactory sedation at mask induction. Similar to satisfactory sedation at parent separation, sedation status at mask induction was evaluated by sedation scores on a four-point sedation scale14,16,17,18 and on a Modified Observer’s Assessment of Alertness/Sedation Scale.19,21,22 There were no differences in satisfactory sedation at mask induction between intranasal dexmedetomidine and premedication with other drugs (RR, 1.25; 95% CI, 0.98 to 1.59; P = 0.08; I2 = 71%) (Fig. 3B). A subgroup analysis also revealed no differences between intranasal dexmedetomidine and intranasal midazolam (RR, 1.14; 95% CI, 0.77 to 1.67; P = 0.51; I2 = 78%) or between intranasal dexmedetomidine and oral midazolam (RR, 1.40; 95% CI, 0.99 to 1.99; P = 0.06; I2 = 71%).
Emergence agitation
The incidence of emergence agitation was extracted from six trials.10,11,13,15,16,18 Emergence agitation was evaluated by a four-point sedation scale,16,18 modified Yale scale,13 Pediatric Anesthesia Emergence Delirium scale,10 or Aonos four-point scale.15 Intranasal dexmedetomidine premedication showed no evidence of reducing emergence agitation when compared with other premedication treatments. (RR, 0.52; 95% CI, 0.24 to 1.13; P = 0.10; I2 = 67%) (Fig. 4A). Also, subgroup analysis showed no difference when dexmedetomidine premedication was compared with intranasal midazolam (RR, 0.70; 95% CI, 0.29 to 1.68; P = 0.42; I2 = 51%), oral midazolam (RR, 0.27; 95% CI, 0.02 to 3.94; P = 0.34; I2 = 87%), and intranasal clonidine (RR, 0.64; 95% CI, 0.31 to 1.31; P = 0.22).
Need for rescue analgesics
Intranasal dexmedetomidine premedication reduced the need for rescue analgesics when compared with other premedication treatments (RR, 0.58; 95% CI, 0.40 to 0.83; P = 0.003; I2 = 0%) (Fig. 4B). Subgroup analysis revealed that intranasal dexmedetomidine premedication was more effective in decreasing postoperative pain than oral midazolam (RR, 0.53; 95% CI, 0.30 to 0.96; P = 0.04; I2 = 0%).
Postoperative nausea and vomiting
The incidence of postoperative nausea and vomiting was extracted from six trials10,11,14,15,16,17 including 496 patients. Patients who received intranasal dexmedetomidine premedication experienced a significantly lower incidence of postoperative nausea and vomiting when compared with other premedication regimes (RR, 0.63; 95% CI, 0.40 to 0.99; P = 0.04; I2= 0%) (Fig. 5A).
Nasal irritation
The incidence of nasal irritation was extracted from three trials10,16,17 including 198 patients. Patients who received intranasal dexmedetomidine premedication experienced a significantly lower incidence of nasal irritation than patients who received intranasal midazolam (RR, 0.05; 95% CI, 0.01 to 0.36; P = 0.003; I2 = 0%) (Fig. 5B).
Time to discharge from the postanesthesia care unit
Four trials10,15,16,22 including 338 patients reported the time to discharge from the postanesthesia care unit. We found no differences between intranasal dexmedetomidine and the other premedication (WMD, 1.2 min; 95% CI, −1.7 to 4.1; P = 0.43; I2 = 94%) (Fig. 5C).
Hemodynamic variables
We extracted SBP data for 167 patients from five trials.14,17,19,21,22 Four trials reported SBP 30 min after premedication, and one trial22 reported SBP at the time of transfer to the operating room. Intranasal dexmedetomidine premedication significantly decreased SBP (WMD, −6.7 mmHg; 95% CI, −10.5 to −2.9; P = 0.0006; I2 = 96%) (Fig. 6A). Heart rate was reported in seven trials13,14,17,18,19,21,22 comprised of 675 patients. Intranasal dexmedetomidine premedication also significantly decreased heart rate (WMD, −6.8 beats·min−1; 95% CI, −11.0 to −3.0; P = 0.002; I2 = 98%) (Fig. 6B). There was no incidence of hypoxia (oxygen saturation < 95%), bradycardia, or hypotension in any group, and these data were extracted from six trials,13,14,16,19,21,22 six trials,11,13,14,16,17,22 and five trails,11,13,16,17,22 respectively.
Sensitivity analysis
We conducted a sensitivity analysis to evaluate how the risk of bias could affect our estimates. The sensitivity analysis of the risk of bias did not affect the results (Table 2). The sensitivity analysis, including only those studies with low risk of bias and satisfactory sedation at parent separation, showed that children receiving intranasal dexmedetomidine were significantly sedated at parent separation (RR, 1.26; 95% CI, 1.06 to 1.75; P = 0.002; I2 = 55%). There were no differences in satisfactory sedation at mask induction between intranasal dexmedetomidine and premedication with other drugs (RR, 1.19; 95% CI, 0.83 to 1.70; P = 0.34; I2 = 80%). Intranasal dexmedetomidine premedication showed no evidence of reducing emergence delirium (RR, 0.47; 95% CI, 0.19 to 1.13; P = 0.09; I2 = 73%).
Quality of the evidence
The GRADE approach was used to assess the quality of each outcome and “Summary of findings” tables were presented (Table 3). As a result, the overall quality of evidence in this meta-analysis was low or moderate. Although the quality of study design was high, most outcomes had problems of inconsistency and imprecision.
Discussion
This meta-analysis revealed that intranasal dexmedetomidine premedication for pediatric patients resulted in more satisfactory sedation at parent separation and reduced the need for rescue analgesics compared with other premedication regimes. Nevertheless, it showed no differences from other intranasal or oral premedicants in satisfactory sedation at mask induction or in the incidence of emergence agitation. Intranasal dexmedetomidine premedication was also associated with a significantly reduced incidence of postoperative nausea and vomiting and nasal irritation compared with other premedication regimes. As for its safety, although children experienced lower SBP and heart rate using intranasal dexmedetomidine premedication, no one needed treatment for hypotension and bradycardia.
Although clinicians frequently use premedication, the ideal agent and route of administration for premedication in children remains uncertain. The most common route for premedication in children is oral administration, but it has low bioavailability.23 Rectal administration often causes pain, could lead to expulsion in young children, and might not be appropriate for older children. An intramuscular approach is not recommended for children because it is invasive.24 The most effective route for premedication in children could be transmucosal, including intranasal, sublingual, and buccal administration, due to the high vascularization of mucosa and its ability to bypass first-pass metabolism.25 Especially for young children, compliance with nasal sedation is more easily attained than oral sedation.26
Thus, intranasal midazolam can be an effective premedication in children. It results in rapid sedation and is commonly administered 30 min before induction or surgery.24 Nevertheless, the sensation of burning and nasal irritation is a disadvantage of this method, and sneezing or coughing caused by the nasal irritation could reduce the effects of nasal premedication.27 In contrast to the nasal irritation often caused by intranasal midazolam, in our meta-analysis, none of the children given intranasal dexmedetomidine premedication exhibited signs of nasal irritation. Moreover, considering the poor bioavailability of orally administered dexmedetomidine, intranasal administration is a more suitable noninvasive route for premedication.7
Although intranasal dexmedetomidine was found to be more effective than intranasal and oral midazolam in achieving satisfactory sedation for separating children and parents, it did not provide satisfactory sedation at mask induction. As described above, sedation with dexmedetomidine has a mechanism similar to natural sleep, with hyperpolarization of norepinephrine receptors in the locus coeruleus.28 Thus, dexmedetomidine leads to sedation without excessive drowsiness, and the resulting sedation is subject to easy and rapid arousal, like natural sleep.6 Therefore, it is not unexpected that patients responded to external stimuli such as mask ventilation.
Dexmedetomidine, a potential alpha-2 agonist, decreases blood pressure and heart rate in a dose-dependent manner.29 Furthermore, rapid injection of dexmedetomidine can have biphasic effects on blood pressure, with temporary increases from a direct α2-adrenoceptor-induced vasoconstrictive response in the peripheral vasculature followed by a lower arterial pressure from a decreased sympathetic outflow.5,30 This biphasic effect on blood pressure can be attenuated by injecting dexmedetomidine slowly.28 In our meta-analysis, children who received intranasal dexmedetomidine as premedication showed lower SBP and heart rate before induction. Nevertheless, no patients in the included trials needed treatment for bradycardia or hypotension. Moreover, small changes (a decrease in heart rate of 6.8 beats·min−1 and a decrease in SBP of 6.7 mmHg) indicate only minor clinical significance as regards these decreases. Because the hemodynamic changes after using dexmedetomidine required no pharmacologic interventions and did not result in any adverse events, dexmedetomidine is considered an appropriate sedative for children.31 Therefore, as long as it is used carefully and avoided for patients at risk of hemodynamic instability, intranasal dexmedetomidine is safe to give as premedication to most children.
The incidence of postoperative nausea and vomiting and the need for rescue analgesics decreased significantly with intranasal dexmedetomidine premedication compared with other treatments. Its antiemetic properties come from the alpha-2 adrenoreceptor agonist effect, which decreases noradrenergic activity by binding to the alpha-2 presynaptic inhibitory receptors in the locus coeruleus in the brain.32 In addition, the analgesic property of dexmedetomidine that reduced postoperative opioid requirements also helped reduce opioid-induced nausea and vomiting.33,34 These facts support the use of intranasal dexmedetomidine as premedication to reduce postoperative nausea and vomiting.
Limitations
This meta-analysis has some limitations. First, we did not prospectively register this review on PRISMA as it was not a requirement for publication at the time we undertook the review. Second, we found significant heterogeneity among studies. Clinical heterogeneity, such as premedication dose, type of intervention, type of surgery, and different age ranges were identified. Because of this clinical heterogeneity, we used random effects models for our meta-analysis. Furthermore, various sedation scales and measurements precluded further synthesis of the data. Third, we tried to synthesize the data on adverse effects; however, we left out some adverse outcomes, e.g., laryngospasm and shivering, due to lack of data. Lastly, we included only a small number of patients in this study. The intervention effects of small clinical trials with incomplete allocation sequence generation, allocation concealment, and double blinding are at risk of being overestimated.35 Although all studies in this meta-analysis used a random allocation method and objectively measured outcome data (e.g., hemodynamic values, postoperative rescue analgesia, and time in the postanesthesia care unit) caution is needed when interpreting our results. Therefore, well-controlled randomized studies are still needed to evaluate the safety of intranasal dexmedetomidine premedication.
In conclusion, this meta-analysis has provided evidence that intranasal dexmedetomidine provides more satisfactory sedation at parent separation than other intranasal or oral premedicants. Additional advantages to intranasal dexmedetomidine premedication include a reduction in the incidence of postoperative nausea and vomiting, nasal irritation, and the need for rescue analgesics. Although lower systolic and mean blood pressure and heart rates were found, those decreases are considered to be of minor clinical significance.
References
Kain ZN, Caldwell-Andrews AA, Krivutza DM, Weinberg ME, Wang SM, Gaal D. Trends in the practice of parental presence during induction of anesthesia and the use of preoperative sedative premedication in the United States, 1995-2002: results of a follow-up national survey. Anesth Analg 2004; 98: 1252-9.
Watson AT, Visram A. Children’s preoperative anxiety and postoperative behaviour. Paediatr Anaesth 2003; 13: 188-204.
Abrams R, Morrison JE, Villasenor A, Hencmann D, Da Fonseca M, Mueller W. Safety and effectiveness of intranasal administration of sedative medications (ketamine, midazolam, or sufentanil) for urgent brief pediatric dental procedures. Anesth Prog 1993; 40: 63-6.
Gyanesh P, Haldar R, Srivastava D, Agrawal PM, Tiwari AK, Singh PK. Comparison between intranasal dexmedetomidine and intranasal ketamine as premedication for procedural sedation in children undergoing MRI: a double-blind, randomized, placebo-controlled trial. J Anesth 2014; 28: 12-8.
Hall JE, Uhrich TD, Barney JA, Arain SR, Ebert TJ. Sedative, amnestic, and analgesic properties of small-dose dexmedetomidine infusions. Anesth Analg 2000; 90: 699-705.
Khan ZP, Ferguson CN, Jones RM. Alpha-2 and imidazoline receptor agonists. Their pharmacology and therapeutic role. Anaesthesia 1999; 54: 146-65.
Anttila M, Penttila J, Helminen A, Vuorilehto L, Scheinin H. Bioavailability of dexmedetomidine after extravascular doses in healthy subjects. Br J Clin Pharmacol 2003; 56: 691-3.
Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions version 5.1.0 [updated March 2011]. The Cochrane Collaboration. Available from URL: http://handbook.cochrane.org (accessed April 2017).
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003; 327: 557-60.
Abdelaziz HM, Bakr RH, Kasem AA. Effect of intranasal dexmedetomidine or intranasal midazolam on prevention of emergence agitation in pediatric strabismus surgery: a randomized controlled study. Egypt J Anaesth 2016; 32: 285-91.
Akin A, Bayram A, Esmaoglu A, et al. Dexmedetomidine vs midazolam for premedication of pediatric patients undergoing anesthesia. Paediatr Anaesth 2012; 22: 871-6.
Lin Y, Chen Y, Huang J, et al. Efficacy of premedication with intranasal dexmedetomidine on inhalational induction and postoperative emergence agitation in pediatric undergoing cataract surgery with sevoflurane. J Clin Anesth 2016; 33: 289-95.
Linares Segovia B, Garcia Cuevas MA, Ramirez Casillas IL, et al. Pre-anesthetic medication with intranasal dexmedetomidine and oral midazolam as an anxiolytic. A clinical trial (Spanish). An Pediatr (Barc) 2014; 81: 226-31.
Mostafa MG, Morsy KM. Premedication with intranasal dexmedetomidine, midazolam and ketamine for children undergoing bone marrow biopsy and aspirate. Egypt J Anesth 2013; 29: 131-5.
Mukherjee A, Das A, Basunia SR, Chattopadhyay S, Kundu R, Bhattacharyya R. Emergence agitation prevention in paediatric ambulatory surgery: a comparison between intranasal dexmedetomidine and clonidine. J Res Pharm Pract 2015; 4: 24-30.
Sheta SA, Al-Sarheed MA, Abdelhalim AA. Intranasal dexmedetomidine vs midazolam for premedication in children undergoing complete dental rehabilitation: a double-blinded randomized controlled trial. Paediatr Anaesth 2014; 24: 181-9.
Singla D, Chaudhary G, Dureja J, Mangla M. Comparison of dexmedetomidine versus midazolam for intranasal premedication in children posted for elevtive surgery: a double-bind, randomised study. South Afr J Anesth Analg 2015; 21: 154-7.
Talon MD, Woodson LC, Sherwood ER, Aarsland A, McRae L, Benham T. Intranasal dexmedetomidine premedication is comparable with midazolam in burn children undergoing reconstructive surgery. J Burn Care Res 2009; 30: 599-605.
Yuen VM, Hui TW, Irwin MG, Yuen MK. A comparison of intranasal dexmedetomidine and oral midazolam for premedication in pediatric anesthesia: a double-blinded randomized controlled trial. Anesth Analg 2008; 106: 1715-21.
Yuen VM, Hui TW, Irwin MG, Yao TJ, Wong GL, Yuen MK. Optimal timing for the administration of intranasal dexmedetomidine for premedication in children. Anaesthesia 2010; 65: 922-9.
Sundaram AM, Mathian VM. A comparative evaluation of intranasal dexmedetomidine and intranasal midazolam for premedication in children: a double blind randomized controlled trial. J Indian Dental Association 2011; 5: 777-81.
Ghali AM, Mahfouz AK, Al-Bahrani M. Preanesthetic medication in children: a comparison of intranasal dexmedetomidine versus oral midazolam. Saudi J Anaesth 2011; 5: 387-91.
Reed MD, Rodarte A, Blumer JL, et al. The single-dose pharmacokinetics of midazolam and its primary metabolite in pediatric patients after oral and intravenous administration. J Clin Pharmacol 2001; 41: 1359-69.
Kogan A, Katz J, Efrat R, Eidelman LA. Premedication with midazolam in young children: a comparison of four routes of administration. Paediatr Anaesth 2002; 12: 685-9.
Rey E, Delaunay L, Pons G, et al. Pharmacokinetics of midazolam in children: comparative study of intranasal and intravenous administration. Eur J Clin Pharmacol 1991; 41: 355-7.
Walbergh EJ, Wills RJ, Eckhert J. Plasma concentrations of midazolam in children following intranasal administration. Anesthesiology 1991; 74: 233-5.
Vivarelli R, Zanotti F, Battaglia D, et al. Premedication with intranasal midazolam in children of various ages (Italian). Minerva Anestesiol 1998; 64: 499-504.
Chrysostomou C, Schmitt CG. Dexmedetomidine: sedation, analgesia and beyond. Expert Opin Drug Metab Toxicol 2008; 4: 619-27.
Talke P, Lobo E, Brown R. Systemically administered alpha2-agonist-induced peripheral vasoconstriction in humans. Anesthesiology 2003; 99: 65-70.
Bloor BC, Ward DS, Belleville JP, Maze M. Effects of intravenous dexmedetomidine in humans. II. Hemodynamic changes. Anesthesiology 1992; 77: 1134-42.
Mason KP, Zgleszewski SE, Prescilla R, Fontaine PJ, Zurakowski D. Hemodynamic effects of dexmedetomidine sedation for CT imaging studies. Paediatr Anaesth 2008; 18: 393-402.
Whittington RA, Virag L. Dexmedetomidine-induced decreases in accumbal dopamine in the rat are partly mediated via the locus coeruleus. Anesth Analg 2006; 102: 448-55.
Gurbet A, Basagan-Mogol E, Turker G, Ugun F, Kaya FN, Ozcan B. Intraoperative infusion of dexmedetomidine reduces perioperative analgesic requirements. Can J Anesth 2006; 53: 646-52.
Lin TF, Yeh YC, Lin FS, et al. Effect of combining dexmedetomidine and morphine for intravenous patient-controlled analgesia. Br J Anaesth 2009; 102: 117-22.
Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta-analyses. Ann Intern Med 2001; 135: 982-9.
Acknowledgement
We sincerely thank the staff members of the Department of Anesthesiology and Pain Medicine at Hanyang University Hospital for their contributions.
Conflicts of interest
None declared.
Editorial responsibility
This submission was handled by Dr. Hilary P. Grocott, Editor-in-Chief, Canadian Journal of Anesthesia.
Author contributions
Jong Hun Jun and Kyu Nam Kim were involved in the study design and writing the article. Jong Hun Jun, Kyu Nam Kim, Ji Yoon Kim, and Shin Me Song were involved in the analysis and interpretation of data. Kyu Nam Kim and Ji Yoon Kim were involved in study selection, data extraction, and assessment of methodological quality.
Financial disclosure
The authors have no financial relationships relevant to this article.
Funding source
There was no external funding.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig 1
Funnel plot for (A) satisfactory sedation at parent separation; (B) satisfactory sedation at mask induction; (C) the incidence of emergence agitation (PDF 51 kb)
Fig 2
Funnel plot for (A) the need for rescue analgesics; (B) the incidence of postoperative nausea and vomiting; (C) the incidence of nasal irritation (PDF 53 kb)
Fig. 3
Funnel plot for (A) the time to discharge from the postanesthesia care unit; (B) systolic blood pressure; (C) heart rate (PDF 49 kb)
Rights and permissions
About this article
Cite this article
Jun, J.H., Kim, K.N., Kim, J.Y. et al. The effects of intranasal dexmedetomidine premedication in children: a systematic review and meta-analysis. Can J Anesth/J Can Anesth 64, 947–961 (2017). https://doi.org/10.1007/s12630-017-0917-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-017-0917-x