Abstract
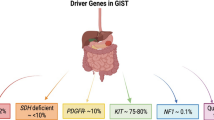
GIST (gastrointestinal stromal tumor) is a rare soft tissue malignancy arising in the gut. It has become well known recently because of the effectiveness of anti-KIT tyrosine kinase inhibitors. From a disease that 10 years ago was only treatable with surgery, now multiple phase 2 and phase 3 trials have identified active first-line systemic therapy, appropriate dosing, an active second-line agent, and established the role of adjuvant therapy after surgery for patients with intermediate- and high-risk tumors. These are accomplishments that took decades to achieve for other more common diseases such as breast cancer or lung cancer. GIST has been the ideal disease system for studying targeted therapy in solid tumors. The progress in treating GIST has come directly from the advances that have been made in the laboratory, understanding the basic biology of tyrosine kinases, the oncogenic activity of c-KIT, and how that enzymatic activity can be inhibited. By studying model diseases such as GIST, we should be able to develop paradigms to treat more common cancers as well.
Similar content being viewed by others
References
Corless CL, Fletcher JA, Heinrich MC. Biology of gastrointestinal stromal tumors. J Clin Oncol. 2004;22:3813–3825.
Nilsson B, Bumming P, Meis-Kindblom JM, et al. Gastrointestinal stromal tumors: the incidence, prevalence, clinical course, and prognostication in the preimatinib mesylate era-a population-based study in western Sweden. Cancer. 2005;103:821–829.
DeMatteo RP, Ballman KV, Antonescu CR, et al. Adjuvant imatinib mesylate after resection of localised, primary gastrointestinal stromal tumour: a randomised, double-blind, placebo-controlled trial. Lancet. 2009;373:1097–1104.
Gold JS, Dematteo RP. Combined surgical and molecular therapy: the gastrointestinal stromal tumor model. Ann Surg. 2006;244:176–184.
Hirota S, Isozaki K, Moriyama Y, et al. Gain-offunction mutations of c-KIT in human gastrointestinal stromal tumors. Science. 1998;279:577–580.
Besmer P, Murphy JE, George PC, et al. A new acute transforming feline retrovirus and relationship of its oncogene v-KIT with the protein kinase gene family. Nature. 1986;320:415–421.
Agaram NP, Wong GC, Guo T, et al. Novel V600E BRAF mutations in imatinib-naive and imatinib-resistant gastrointestinal stromal tumors. Genes Chromosomes Cancer. 2008;47:853–859.
Segal NH, Pavlidis P, Antonescu CR, et al. Classification and subtype prediction of adult soft tissue sarcoma by functional genomics. Am J Pathol. 2003;163:691–700.
Tuveson DA, Willis NA, Jacks T, et al. STI571 inactivation of the gastrointestinal stromal tumor c-KIT oncoprotein: biological and clinical implications. Oncogene. 2001;20:5054–5058.
Joensuu H, Roberts PJ, Sarlomo-Rikala M, et al. Effect of the tyrosine kinase inhibitor STI571 in a patient with a metastatic gastrointestinal stromal tumor. N Engl J Med. 2001;344:1052–1056.
Demetri GD, von Mehren M, Blanke CD, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002;347:472–480.
Verweij J, van Oosterom A, Blay JY, et al. Imatinib mesylate (STI-571 Glivec, Gleevec) is an active agent for gastrointestinal stromal tumours, but does not yield responses in other soft-tissue sarcomas that are unselected for a molecular target. Results from an EORTC Soft Tissue and Bone Sarcoma Group phase II study. Eur J Cancer. 2003;39:2006–2011.
Blanke CD, Demetri GD, von Mehren M, et al. Long-term results from a randomized phase II trial of standard- versus higher-dose imatinib mesylate for patients with unresectable or metastatic gastrointestinal stromal tumors expressing KIT. J Clin Oncol. 2008;26:620–625.
Ridruejo E, Cacchione R, Villamil AG, Marciano S, Gadano AC, Mando OG. Imatinib-induced fatal acute liver failure. World J Gastroenterol. 2007;13:6608–6111.
Kerkela R, Grazette L, Yacobi R, et al. Cardiotoxicity of the cancer therapeutic agent imatinib mesylate. Nat Med. 2006;12:908–916.
Verweij J, Casali PG, Kotasek D, et al. Imatinib does not induce cardiac left ventricular failure in gastrointestinal stromal tumours patients: analysis of EORTC-ISG-AGITG study 62005. Eur J Cancer. 2007;43:974–978.
Blanke CD, Rankin C, Demetri GD, et al. Phase III randomized, intergroup trial assessing imatinib mesylate at two dose levels in patients with unresectable or metastatic gastrointestinal stromal tumors expressing the KIT receptor tyrosine kinase: S0033. J Clin Oncol. 2008;26:626–632.
Zalcberg JR, Verweij J, Casali PG, et al. Outcome of patients with advanced gastro-intestinal stromal tumours crossing over to a daily imatinib dose of 800 mg after progression on 400 mg. Eur J Cancer. 2005;41:1751–1757.
Demetri GD, Benjamin RS, Blanke CD, et al. NCCN task force report: management of patients with gastrointestinal stromal tumor (GIST) - update of the NCCN clinical practice guidelines. J Natl Compr Canc Netw. 2007;5(suppl. 2):S1–S29. Quiz S30.
Heinrich MC, Owzar K, Corless CL, et al. Correlation of kinase genotype and clinical outcome in the North American Intergroup phase III trial of imatinib mesylate for treatment of advanced gastrointestinal stromal tumor: CALGB 150105 Study by Cancer and Leukemia Group B and Southwest Oncology Group. J Clin Oncol. 2008;26:5360–5367.
Demetri GD, van Oosterom AT, Garrett CR, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet. 2006;368:1329–1338.
Heinrich MC, Maki RG, Corless CL, et al. Primary and secondary kinase genotypes correlate with the biological and clinical activity of sunitinib in imatinib-resistant gastrointestinal stromal tumor. J Clin Oncol. 2008;26:5352–5359.
Abrams TJ, Lee LB, Murray LJ, Pryer NK, Cherrington JM. SU11248 inhibits KIT and platelet-derived growth factor receptor beta in preclinical models of human small cell lung cancer. Mol Cancer Ther. 2003;2:471–478.
Joensuu H, De Braud F, Coco P, et al. Phase II, openlabel study of PTK787/ZK222584 for the treatment of metastatic gastrointestinal stromal tumors resistant to imatinib mesylate. Ann Oncol. 2008;19:173–177.
Benjamin RS, Schuetze SM, Blay JY, et al. Initial results of a multicenter, single-arm phase 2 study of AMG 706, an oral multikinase inhibitor, for the treatment of advanced imatinib-resistant gastrointestinal stromal tumors (GIST). Presented at: the Connective Tissue Oncology Society annual meeting; November 2–4, 2006; Venice, Italy. Presentation no. 641.
Wiebe L, Kasza KE, Maki R, et al. Activity of sorafenib (SOR) in patients (pts) with imatinib (IM) and sunitinib (SU)-resistant (RES) gastrointestinal stromal tumors (GIST): a phase II trial of the University of Chicago Phase II Consortium. J Clin Oncol. 2008;26(suppl.). Abstract 10502.
Blay JY, Casali P, Reichard P, et al. A phase I study of nilotinib alone and in combination with imatinib in patients with imatinib-resistant gastrointestinal stromal tumors (GIST): study update. J Clin Oncol. 2008;26(suppl.). Abstract 10553.
Bauer S, Yu LK, Demetri GD, Fletcher JA. Heat shock protein 90 inhibition in imatinib-resistant gastrointestinal stromal tumor. Cancer Res. 2006;66:9153–9161.
Rossi F, Ehlers I, Agosti V, et al. Oncogenic Kit signaling and therapeutic intervention in a mouse model of gastrointestinal stromal tumor. Proc Natl Acad Sci U S A. 2006;103:12843–12848.Í
Sambol EB, Ambrosini G, Geha RC, et al. Flavopiridol targets c-KIT transcription and induces apoptosis in gastrointestinal stromal tumor cells. Cancer Res. 2006;66:5858–5866.
Raut CP, Posner M, Desai J, et al. Surgical management of advanced gastrointestinal stromal tumors after treatment with targeted systemic therapy using kinase inhibitors. J Clin Oncol. 2006;24:2325–2331.
DeMatteo RP, Maki RG, Singer S, Gonen M, Brennan MF, Antonescu CR. Results of tyrosine kinase inhibitor therapy followed by surgical resection for metastatic gastrointestinal stromal tumor. Ann Surg. 2007;245:347–352.
Hurwitz J, Constantinidou A, Saran F, Scurr M, Rahman A, Judson I. The role of radiotherapy in metastatic gastrointestinal stromal tumor (GIST). Proceedings of the Connective Tissue Oncology Society. 2008;14. Abstract 35023.
Kobayashi K, Gupta S, Trent JC, et al. Hepatic artery chemoembolization for 110 gastrointestinal stromal tumors: response, survival, and prognostic factors. Cancer. 2006;107:2833–2841.
Blay JY, Le Cesne A, Ray-Coquard I, et al. Prospective multicentric randomized phase III study of imatinib in patients with advanced gastrointestinal stromal tumors comparing interruption versus continuation of treatment beyond 1 year: the French Sarcoma Group. J Clin Oncol. 2007;25:1107–1113.
Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2006. CA Cancer J Clin. 2006;56:106–130.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
D’Adamo, D. Advances in the treatment of gastrointestinal stromal tumor. Adv Therapy 26, 826–837 (2009). https://doi.org/10.1007/s12325-009-0067-9
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-009-0067-9