Abstract
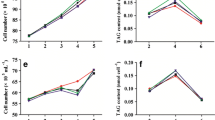
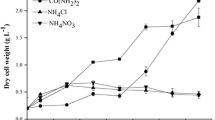
Microalgae are attracting much attention as superior biodiesel producers. In particular, under stressful conditions, they accumulate organic compounds consisting entirely of carbon and hydrogen. The aim of this work was to increase intracellular fatty acid content in Dunaliella tertiolecta (Chlorophyceae), Nannochloropsis oculata (Eustigmatophyceae), and Porphyridium cruentum (Rhodophyceae) using a combination of nitrogen starvation and chemical inhibitors of carbohydrate biosynthesis. These microalgae were subjected to nitrogen starvation and their physiological changes were then observed over time. In D. tertiolecta, no significant change in total fatty acid content was detected on day 3.5 relative to the initial total fatty acid content (day 0), while total carbohydrate content dramatically increased as the nitrogen starvation period was extended. In N. oculata, total fatty acid content rapidly increased, reaching up to nearly 40% of the DCW at day 3.5. However, total carbohydrate content exhibited a gradual reduction throughout the experiment. In P. cruentum, total carbohydrate content increased up to 43% of DCW on day 3.5 and total fatty acid content increased slightly under nitrogen depletion. These data suggest that different eukaryotic microalgae use different storage products under stressful conditions. Among the three strains, D. tertiolecta showed decreased total carbohydrate content and enhanced total fatty acid content following inhibition of carbohydrate synthesis by dichlorophenyl dimethylurea and cyclohexane diamine tetra acetic acid. The results demonstrate the possibility of furthering our understanding of the fatty acid and carbohydrate biosynthesis metabolic network that responds to environmental changes in microalgae.
Similar content being viewed by others
References
Mata, T. M., A. A. Martins, and N. S. Caetano (2010) Microalgae for biodiesel production and other applications: A review. Renew. Sust. Energ. Rev. 14: 217–232.
Liao, J. C., L. Mi, S. Pontrelli, and S. Luo (2016) Fuelling the future: Microbial engineering for the production of sustainable biofuels. Nat. Rev. Microbiol. 14: 288–304.
Campbell, P. K., T. Beer, and D. Batten (2011) Life cycle assessment of biodiesel production from microalgae in ponds. Bioresour. Technol. 102: 50–56.
Pancha, I., K. Chokshi, B. George, T. Ghosh, C. Paliwal, R. Maurya, and S. Mishra (2014) Nitrogen stress triggered biochemical and morphological changes in the microalgae Scenedesmus sp. CCNM 1077. Bioresour. Technol. 156: 146–154.
Adams, C., V. Godfrey, B. Wahlen, L. Seefeldt, and B. Bugbee (2013) Understanding precision nitrogen stress to optimize the growth and lipid content tradeoff in oleaginous green microalgae. Bioresour. Technol. 131: 188–194.
Dalay, M. C. (2007) Introduction to the algal world. pp. xix In: J. Seck (ed.). Algae and cyanobacteria in extreme environments. Springer, Dordrecht, The Netherlands.
Chen, M., H. Tang, H. Ma, T. C. Holland, K. Y. S. Ng, and S. O. Salley (2011) Effect of nutrients on growth and lipid accumulation in the green algae Dunaliella tertiolecta. Bioresour. Technol. 102: 1649–1655.
Söyler, N., J. L. Goldfarb, S. Ceylan, and M. T. Saçan (2017) Renewable fuels from pyrolysis of Dunaliella tertiolecta: An alternative approach to biochemical conversions of microalgae. Energy. 120: 907–914.
Bermejo Román, R., J. M. Alvárez-Pez, F. G. Acién Fernández, and E. Molina Grima (2002) Recovery of pure B-phycoerythrin from the microalga Porphyridium cruentum. J. Biotechnol. 93: 73–85.
Kavitha, M. D., S. Kathiresan, S. Bhattacharya, and R. Sarada (2016) Culture media optimization of Porphyridium purpureum: production potential of biomass, total lipids, arachidonic and eicosapentaenoic acid. J. Food Sci. Technol. 153: 2270–2278.
Lutzu, G. A., L. Zhang, Z. Zhang, and T. Liu (2017) Feasibility of attached cultivation for polysaccharides production by Porphyridium cruentum. Bioprocess Biosyst. Eng. 40: 73–83.
Rodolfi, L., G. Chini Zittelli, N. Bassi, G. Padovani, N. Biondi, G. Bonini, and M. R. Tredici (2009) Microalgae for oil: Strain selection, induction of lipid synthesis and outdoor mass cultivation in a low-cost photobioreactor. Biotechnol. Bioeng. 102: 100–112.
Li, Y., D. Han, G. Hu, D. Dauvillee, M. Sommerfeld, S. Ball, and Q. Hu (2010) Chlamydomonas starchless mutant defective in ADP-glucose pyrophosphorylase hyper-accumulates triacylglycerol. Metab. Eng. 12: 387–391.
Daboussi, F., S. Leduc, A. Maréchal, G. Dubois, V. Guyot, C. Perez-Michaut, A. Amato, A. Falciatore, A. Juillerat, M. Beurdeley, D. F. Voytas, L. Cavarec, and P. Duchateau (2014) Genome engineering empowers the diatom Phaeodactylum tricornutum for biotechnology. Nat. Commun. 5: 3831
Li, Y., D. Han, M. Sommerfeld, and Q. Hu (2011) Photosynthetic carbon partitioning and lipid production in the oleaginous microalga Pseudochlorococcum sp. (Chlorophyceae) under nitrogen-limited conditions. Bioresour. Technol. 102: 123–129.
Recht, L., A. Zarka, and S. Boussiba (2012) Patterns of carbohydrate and fatty acid changes under nitrogen starvation in the microalgae Haematococcus pluvialis and Nannochloropsis sp. Appl. Microbiol. Biotechnol. 94: 1495–1503.
Jones, L. W. and J. Myers (1963) A common link between photosynthesis and respiration in a blue-green alga. Nature 199: 670–672.
Lee, H. S., Z. H. Kim, H. Park, and C. G. Lee (2016) Specific light uptake rates can enhance astaxanthin productivity in Haematococcus lacustris. Bioproc. Biosyst. Eng. 39: 815–823.
Lee, H. S., M. W. Seo, Z. H. Kim, and C. G. Lee (2006) Determining the best specific light uptake rates for the lumostatic cultures in bubble column photobioreactors. Enzyme Microb. Technol. 39: 447–452.
Dubois, M., K. A. Gilles, J. K. Hamilton, P. Rebers, and F. Smith (1956) Colorimetric method for determination of sugars and related substances. Anal. Chem. 28: 350–356.
Gonen-Zurgil, Y., Y. Carmeli-Schwartz, and A. Sukenik (1996) Selective effect of the herbicide DCMU on unicellular algae—a potential tool to maintain monoalgal mass culture of Nannochloropsis. J. Appl. Phycol. 8: 415–419.
Bury, N., G. Flik, F. Eddy, and G. Codd (1996) The effects of cyanobacteria and the cyanobacterial toxin microcystin-LR on Ca2+ transport and Na+/K+-ATPase in tilapia gills. J. Exp. Biol. 199: 1319–1326.
Geider, R. J., H. L. MacIntyre, and T. M. Kana (1998) A dynamic regulatory model of phytoplanktonic acclimation to light, nutrients, and temperature. Limnol. Oceanogr. 43: 679–694.
Zhu, S., W. Huang, J. Xu, Z. Wang, J. Xu, and Z. Yuan (2014) Metabolic changes of starch and lipid triggered by nitrogen starvation in the microalga Chlorella zofingiensis. Bioresour. Technol. 152: 292–298.
Markou, G., I. Angelidaki, and D. Georgakakis (2012) Microalgal carbohydrates: An overview of the factors influencing carbohydrates production, and of main bioconversion technologies for production of biofuels. Appl. Microbiol. Biotechnol. 96: 631–645.
Scibilia, L., L. Girolomoni, S. Berteotti, A. Alboresi, and M. Ballottari (2015) Photosynthetic response to nitrogen starvation and high light in Haematococcus pluvialis. Algal Res. 12: 170–181.
Becker, E. W. (1994) Microalgae: Biotechnology and Microbiology. pp. 18–24. Cambridge University Press, Cambridge, UK.
Su, C.-H., L.-J. Chien, J. Gomes, Y.-S. Lin, Y.-K. Yu, J.-S. Liou, and R.-J. Syu (2011) Factors affecting lipid accumulation by Nannochloropsis oculata in a two-stage cultivation process. J. Appl. Phycol. 23: 903–908.
Roessler, P. G. (1990) Environmental control of glycerolipid metabolism in microalgae: Commercial implications and future research directions. J. Phycol. 26: 393–399.
Kuchitsu, K., M. Tsuzuki, and S. Miyachi (1988) Changes of starch localization within the chloroplast induced by changes in CO2 concentration during growth of Chlamydomonas reinhardtii: Independent regulation of pyrenoid starch and stroma starch. Plant Cell Physiol. 29: 1269–1278.
Roeben, A., J. M. Plitzko, R. Körner, U. M. K. Böttcher, K. Siegers, M. Hayer-Hartl, and A. Bracher (2006) Structural basis for subunit assembly in UDP-glucose pyrophosphorylase from Saccharomyces cerevisiae. J. Mol. Biol. 364: 551–560.
Patron, N. J. and P. J. Keeling (2005) Common evolutionary origin of starch biosynthetic enzymes in green and red algae. J. Phycol. 41: 1131–1141.
Iglesias, A. A., Y. Charng, S. Ball, and J. Preiss (1994) Characterization of the kinetic, regulatory, and structural properties of ADP-glucose pyrophosphorylase from Chlamydomonas reinhardtii. Plant Physiol. 104: 1287–1294.
Klein, U. (1987) Intracellular carbon partitioning in Chlamydomonas reinhardtii. Plant Physiol. 85: 892–897.
Kleczkowski, L. A., M. Geisler, I. Ciereszko, and H. Johansson (2004) UDP-glucose pyrophosphorylase. An old protein with new tricks. Plant Physiol. 134: 912–918.
Chan, C. X., E. C. Yang, T. Banerjee, H. S. Yoon, P. T. Martone, J. M. Estevez, and D. Bhattacharya (2011) Red and green algal monophyly and extensive gene sharing found in a rich repertoire of red algal genes. Curr. Biol. 21: 328–333.
Radakovits, R., R. E. Jinkerson, S. I. Fuerstenberg, H. Tae, R. E. Settlage, J. L. Boore, and M. C. Posewitz (2012) Draft genome sequence and genetic transformation of the oleaginous alga Nannochloropsis gaditana. Nat. Commun. 3: 686.
Author information
Authors and Affiliations
Corresponding author
Additional information
These two author’s contributed equally to this work.
Rights and permissions
About this article
Cite this article
Hong, SJ., Park, Y.S., Han, MA. et al. Enhanced production of fatty acids in three strains of microalgae using a combination of nitrogen starvation and chemical inhibitors of carbohydrate synthesis. Biotechnol Bioproc E 22, 60–67 (2017). https://doi.org/10.1007/s12257-016-0575-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12257-016-0575-9