Abstract
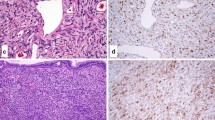
Sinonasal hemangiopericytoma-like tumors(SNHPC)(glomangiopericytomas)were originally conceived as histologically similar to, but biologically distinct from, their soft tissue counterparts. Re-evaluation of “hemangiopericytiomas” has determined that SNHPC (glomangiopericytomas) represent bona-fide pericyte-derived tumors, whereas most soft tissue neoplasms previously designated as hemangiopericytomas represent cellular variants of solitary fibrous tumors or other lesions with a hemangiopericytomalike growth pattern. We present an interesting case of a woman with SNHPC (glomangiopericytomas) causing oncogenic osteomalacia, and discuss the recent advances in our understanding of phosphaturic mesenchymal tumors. This particular case is an example of “Striking Pathology Gold”—a situation where the pathologist actively guides the diagnostic process, and witnesses its repercussions. “Striking Pathology Gold” may be a rare event in one’s career. However it serves to remind us of our place in the world as physicians. Working behind the scenes, we quietly change the course of countless individual destinies for the better.












Similar content being viewed by others
References
Stout AP, Murray MR. Hemangiopericytoma: a vascular tumor featuring Zimmermann’s pericytes. Ann Surg. 1942;116:26–33.
Armulik A, Genové G, Betsholtz C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell. 2011;21:193–215.
Compagno J, Hyams VJ. Hemangiopericytoma-like intranasal tumors. A clinicopathologic study of 23 cases. Am J Clin Pathol. 1976;66:672–83.
Fletcher CD. The evolving classification of soft tissue tumours: an update based on the new WHO classification. Histopathology. 2006;48:3–12.
Thompson LD, Miettinen M, Wenig BM. Sinonasal-type hemangiopericytoma: a clinicopathologic and immunophenotypic analysis of 104 cases showing perivascular myoid differentiation. Am J Surg Pathol. 2003;27:737–49.
Dandekar M, McHugh JB. Sinonasal glomangiopericytoma: case report with emphasis on the differential diagnosis. Arch Pathol Lab Med. 2010;134:1444–9.
Wilson T, Hellquist HB, Ray S, Pickles J. Intranasal myopericytoma. A tumour with perivascular myoid differentiation: the changing nomenclature for haemangiopericytoma. J Laryngol Otol. 2007;121:786–9.
Kuo FY, Lin HC, Eng HL, Huang CC. Sinonasal hemangiopericytoma-like tumor with true pericytic myoid differentiation: a clinicopathologic and immunohistochemical study of five cases. Head Neck. 2005;27:124–9.
Tse LL, Chan JK. Sinonasal haemangiopericytoma-like tumour: a sinonasal glomus tumour or a haemangiopericytoma? Histopathology. 2002;40:510–7.
Hansen T, Katenkamp K, Katenkamp D. D2–40 staining in sinonasal-type hemangiopericytoma–further evidence of distinction from conventional hemangiopericytoma and solitary fibrous tumor. Virchows Arch. 2006;448:459–62.
Catalano PJ, Brandwein M, Shah DK, Urken ML, Lawson W, Biller HF. Sinonasal hemangiopericytomas: a clinicopathologic and immunohistochemical study of seven cases. Head Neck. 1996;18:42–53.
Weidner N, Bar RS, Weiss D, Strottmann MP. Neoplastic pathology of oncogenic osteomalacia/rickets. Cancer. 1985;55:1691–705.
Weidner N, Santa Cruz D. Phosphaturic mesenchymal tumors. A polymorphous group causing osteomalacia or rickets. Cancer. 1987;59:1442–54.
Weidner N. Review and update: oncogenic osteomalacia-rickets. Ultrastruct Pathol. 1991;15:317–33.
Chong WH, Molinolo AA, Chen CC, Collins MT. Tumor-induced osteomalacia. Endocr Relat Cancer. 2011;18:53–77.
Farrow EG, White KE. Tumor-induced osteomalacia. Expert Rev Endocrinol Metab. 2009;4:435–42.
Weiss D, Bar RS, Weidner N, Wener M, Lee F. Oncogenic osteomalacia: strange tumours in strange places. Postgrad Med J. 1985;61:349–55.
Folpe AL, Fanburg-Smith JC, Billings SD, Bisceglia M, Bertoni F, Cho JY, Econs MJ, Inwards CY, Jan de Beur SM, Mentzel T, Montgomery E, Michal M, Miettinen M, Mills SE, Reith JD, OConnell JX, Rosenberg AE, Rubin BP, DE Sweet, Vinh TN, Wold LE, Wehrli BM, White KE, Zaino RJ, Weiss SW. Most osteomalacia-associated mesenchymal tumors are a single histopathologic entity: an analysis of 32 cases and a comprehensive review of the literature. Am J Surg Pathol. 2004;28:1–30.
Komínek P, Stárek I, Geierová M, Matoušek P, Zeleník K. Phosphaturic mesenchymal tumour of the sinonasal area: case report and review of the literature. Head Neck Oncol. 2011;3:16–20.
Shelekhova KV, Kazakov DV, Michal M. Sinonasal phosphaturic mesenchymal tumor (mixed connective tissue variant): report of 2 cases. Am J Surg Pathol. 2010;34:596–7.
Inokuchi G, Tanimoto H, Ishida H, Sugimoto T, Yamauchi M, Miyauchi A, Nibu K. A paranasal tumor associated with tumor-induced osteomalacia. Laryngoscope. 2006;116:1930–3.
Woo VL, Landesberg R, Imel EA, Singer SR, Folpe AL, Econs MJ, Kim T, Harik LR, Jacobs TP. Phosphaturic mesenchymal tumor, mixed connective tissue variant, of the mandible: report of a case and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108:925–32.
Pedrazzoli M, Colletti G, Ferrari M, Rossetti G, Moneghini L, Autelitano L. Mesenchymal phosphaturic neoplasm in the maxillary sinus: a case report. Int J Oral Maxillofac Surg. 2010;39:1027–32.
Battoo AJ, Salih S, Unnikrishnan AG, Jojo A, Bahadur S, Iyer S, Kuriakose MA. Oncogenic osteomalacia from nasal cavity giant cell tumor. Head Neck. 2010 Dec 9. [Epub ahead of print] PubMed PMID: 21154616.
Parshwanath HA, Kulkarni PR, Rao R, Joshi SK, Patil P. Phosphaturic mesenchymal tumor of ethmoid sinus. Indian J Pathol Microbiol. 2010;53:384–5.
David K, Revesz T, Kratimenos G, Krausz T, Crockard HA. Oncogenic osteomalacia associated with a meningeal phosphaturic mesenchymal tumor. J Neurosurg. 1996;84:288–92.
Peterson NR, Summerlin DJ, Cordes SR. Multiple phosphaturic mesenchymal tumors associated with oncogenic osteomalacia: case report and review of the literature. Ear Nose Throat J. 2010;89:11–5.
Gonzalez-Compta X, Mañós-Pujol M, Foglia-Fernandez M, Peral E, Condom E, Claveguera T, Dicenta-Sousa M. Oncogenic osteomalacia: case report and review of head and neck associated tumours. J Laryngol Otol. 1998;112:389–92.
Koriyama N, Nishimoto K, Kodama T, Nakazaki M, Kurono Y, Yoshida H, Tei C. Oncogenic osteomalacia in a case with a maxillary sinus mesenchymal tumor. Am J Med Sci. 2006;332:142–7.
Shelekhova KV, Kazakov DV, Hes O, Treska V, Michal M. Phosphaturic mesenchymal tumor (mixed connective tissue variant): a case report with spectral analysis. Virchows Arch. 2006;448:232–5.
Nuovo MA, Dorfman HD, Sun CC, Chalew SA. Tumor-induced osteomalacia and rickets. Am J Surg Pathol. 1989;13:588–99.
Ghosh S, Sinha R, Bandyopadhyay R, Malhotra M. Oncogenous osteomalacia. J Cancer Res Ther. 2009;5:210–2.
Savage CR, Zimmer LA. Oncogenic osteomalacia from pterygopalatine fossa mass. J Laryngol Otol. 2009;123:1052–4.
Wilkins GE, Granleese S, Hegele RG, Holden J, Anderson DW, Bondy GP. Oncogenic osteomalacia: evidence for a humoral phosphaturic factor. J Clin Endocrinol Metab. 1995;80:1628–34.
Ohashi K, Ohnishi T, Ishikawa T, Tani H, Uesugi K, Takagi M. Oncogenic osteomalacia presenting as bilateral stress fractures of the tibia. Skeletal Radiol. 1999;28:46–8.
Sandhu FA, Martuza RL. Craniofacial hemangiopericytoma associated with oncogenic osteomalacia: case report. J Neurooncol. 2000;46:241–7.
Fuentealba C, Pinto D, Ballesteros F, Pacheco D, Boettiger O, Soto N, Fernandez W, Gabler F, Gonzales G, Reginato AJ. Oncogenic hypophosphatemic osteomalacia associated with a nasal hemangiopericytoma. J Clin Rheumatol. 2003;9:373–9.
Kurien R, Manipadam MT, Rupa V. Oncogenic osteomalacia in a patient with an ethmoid sinus tumour. J Laryngol Otol. 2010;124:799–803.
Chacko V, Joseph B. Osteomalacia associated with haemangiopericytoma. J Indian Med Assoc. 1981;76:173–5.
Peters KB, McLendon R, Morse MA, Vredenburgh JJ. Treatment of recurrent intracranial hemangiopericytoma with SRC-related tyrosine kinase targeted therapy: a case report. Case Rep Oncols. 2010;3:93–7.
Bielesz B. Emerging role of a phosphatonin in mineral homeostasis and its derangements. Eur J Clin Invest. 2006;36:34–42.
Riminucci M, Collins MT, Fedarko NS, Cherman N, Corsi A, White KE, Waguespack S, Gupta A, Hannon T, Econs MJ, Bianco P, Gehron Robey P. FGF-23 in fibrous dysplasia of bone and its relationship to renal phosphate wasting. J Clin Invest. 2003;112:683–92.
Urakawa I, Yamazaki Y, Shimada T, Iijima K, Hasegawa H, Okawa K, Fujita T, Fukumoto S, Yamashita T. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature. 2006;444:770–4.
Perwad F, Zhang MY, Tenenhouse HS, Portale AA. Fibroblast growth factor 23 impairs phosphorus and vitamin D metabolism in vivo and suppresses 25-hydroxyvitamin D-1alpha-hydroxylase expression in vitro. Am J Physiol Renal Physiol. 2007;293:1577–83.
Gupta A, Winer K, Econs MJ, Marx SJ, Collins MT. FGF-23 is elevated by chronic hyperphosphatemia. J Clin Endocrinol Metab. 2004;89:4489–92.
Shimada T, Mizutani S, Muto T, Yoneya T, Hino R, Takeda S, Takeuchi Y, Fujita T, Fukumoto S, Yamashita T. Cloning and characterization of FGF23 as a causative factor of tumor-induced osteomalacia. Proc Natl Acad Sci USA. 2001;98:6500–5.
Ben-Dov IZ, Galitzer H, Lavi-Moshayoff V, Goetz R, Kuro-o M, Mohammadi M, Sirkis R, Naveh-Many T, Silver J. The parathyroid is a target organ for FGF23 in rats. J Clin Invest. 2007;117:4003–8.
Jonsson KB, Zahradnik R, Larsson T, White KE, Sugimoto T, Imanishi Y, Yamamoto T, Hampson G, Koshiyama H, Ljunggren O, Oba K, Yang IM, Miyauchi A, Econs MJ, Lavigne J, Jüppner H. Fibroblast growth factor 23 in oncogenic osteomalacia and X-linked hypophosphatemia. N Engl J Med. 2003;348:1656–63.
Yamazaki Y, Okazaki R, Shibata M, Hasegawa Y, Satoh K, Tajima T, Takeuchi Y, Fujita T, Nakahara K, Yamashita T, Fukumoto S. Increased circulatory level of biologically active full-length FGF-23 in patients with hypophosphatemic rickets/osteomalacia. J Clin Endocrinol Metab. 2002;87:4957–60.
Hu FK, Yuan F, Jiang CY, Lv DW, Mao BB, Zhang Q, Yuan ZQ, Wang Y. Tumor-induced osteomalacia with elevated fibroblast growth factor 23: a case of phosphaturic mesenchymal tumor mixed with connective tissue variants and review of the literature. Chin J Cancer. 2011;30:794–804.
Levy-Litan V, Hershkovitz E, Avizov L, Leventhal N, Bercovich D, Chalifa-Caspi V, Manor E, Buriakovsky S, Hadad Y, Goding J, Parvari R. Autosomal-recessive hypophosphatemic rickets is associated with an inactivation mutation in the ENPP1 gene. Am J Hum Genet. 2010;86:273–8.
Farrow EG, Davis SI, Ward LM, Summers LJ, Bubbear JS, Keen R, Stamp TC, Baker LR, Bonewald LF, White KE. Molecular analysis of DMP1 mutants causing autosomal recessive hypophosphatemic rickets. Bone. 2009;44:287–94.
Martin A, Liu S, David V, Li H, Karydis A, Feng JQ, Quarles LD. Bone proteins PHEX and DMP1 regulate fibroblastic growth factor Fgf23 expression in osteocytes through a common pathway involving FGF receptor (FGFR) signaling. FASEB J. 2011;25:2551–62.
De Beur SM, Finnegan RB, Vassiliadis J, Cook B, Barberio D, Estes S, Manavalan P, Petroziello J, Madden SL, Cho JY, Kumar R, Levine MA, Schiavi SC. Tumors associated with oncogenic osteomalacia express genes important in bone and mineral metabolism. J Bone Miner Res. 2002;17:1102–10.
Habra MA, Jimenez C, Huang SC, Cote GJ, Murphy WA Jr, Gagel RF, Hoff AO. Expression analysis of fibroblast growth factor-23, matrix extracellular phosphoglycoprotein, secreted frizzled-related protein-4, and fibroblast growth factor-7: identification of fibroblast growth factor-23 and matrix extracellular phosphoglycoprotein as major factors involved in tumor-induced osteomalacia. Endocr Pract. 2008;14:1108–14.
Cotant CL, Rao PS. Elevated fibroblast growth factor 23 in a patient with metastatic prostate cancer and hypophosphatemia. Am J Kidney Dis. 2007;50:1033–6.
Imel EA, Econs MJ. Fibrous dysplasia, phosphate wasting and fibroblast growth factor 23. Pediatr Endocrinol Rev. 2007;4:434–9.
Ben-Baruch D, Ziv Y, Sandbank J, Wolloch Y. Oncogenic osteomalacia induced by schwannoma in a patient with neurofibromatosis. Eur J Surg Oncol. 1994;20:57–61.
Chadha M, Singh AP, Singh AP. Hypophosphataemic osteomalacia in neurofibromatosis. Acta Orthop Belg. 2009;75:847–50.
Sprecher E. Familial tumoral calcinosis: from characterization of a rare phenotype to the pathogenesis of ectopic calcification. J Invest Dermatol. 2010;130:652–60.
Xu YY, Luo XM, Zhou SH, Zheng ZJ. CD34-positive expression in benign nasal glomus tumour: two case reports and a literature review. J Int Med Res. 2010;38:2169–77.
Ganly I, Patel SG, Stambuk HE, Coleman M, Ghossein R, Carlson D, Edgar M, Shah JP. Solitary fibrous tumors of the head and neck: a clinicopathologic and radiologic review. Arch Otolaryngol Head Neck Surg. 2006;132:517–25.
Papadakis I, Koudounarakis E, Haniotis V, Karatzanis A, Velegrakis G. Atypical solitary fibrous tumor of the nose and maxillary sinus. Head Neck. 2011;. doi:10.1002/hed.21909.
Nai GA, Ramalho Neto GC. Solitary fibrous tumor of the nasal cavity. Braz J Otorhinolaryngol. 2009;75:769.
Takasaki K, Watanabe T, Hayashi T, Kinoshita N, Kumagami H, Takahashi H. Solitary fibrous tumor arising from the sphenoid sinus. Case Report Med. 2009; 316042.
Furze AD, Peng Y, Myers LL. Pathology case quiz 2. Solitary fibrous tumor of the nasal cavity and ethmoid sinus with intracranial extension. Arch Otolaryngol Head Neck Surg. 2008;134(334):336–7.
Corina L, Volante M, Carconi M, Contucci AM. An unusual solitary fibrous tumor after sphenoethmoidectomy. Otolaryngol Head Neck Surg. 2006;134:1063–5.
Martin H, Kay WN, Werner P. Angiomatous meningioma—a clinicopathologic study of 38 cases. Am J Surg Pathol. 2004;28:390–3.
Ambrosini-Spaltro A, Eusebi V. Meningeal hemangiopericytomas and hemangiopericytoma/solitary fibrous tumors of extracranial soft tissues: a comparison. Virchows Arch. 2010;456:343–54.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Brandwein-Gensler, M., Siegal, G.P. Striking Pathology Gold: A Singular Experience with Daily Reverberations: Sinonasal Hemangiopericytoma (Glomangiopericytoma) and Oncogenic Osteomalacia. Head and Neck Pathol 6, 64–74 (2012). https://doi.org/10.1007/s12105-012-0337-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12105-012-0337-8