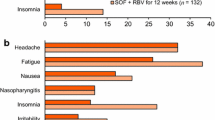
Abstract
Background
The standard-of-care regimen for chronic hepatitis C virus (HCV) infection in Korea, pegylated-interferon-alpha plus ribavirin, is poorly tolerated. Ledipasvir/sofosbuvir is a two-drug, fixed-dose combination tablet approved in the USA, European Union, and Japan for chronic genotype 1 HCV infection.
Methods
This single-arm, phase IIIb study (NCT02021656) investigated the efficacy and safety of ledipasvir/sofosbuvir fixed-dose combination tablet for 12 weeks in treatment-naïve and treatment-experienced Korean patients chronically infected with genotype 1 HCV with or without compensated cirrhosis.
Results
The proportion of patients with sustained virologic response 12 weeks after treatment discontinuation (SVR12) was 99 % (92/93), with rates of 100 % (46/46) and 98 % (46/47) in treatment-naïve and treatment-experienced patients, respectively. There were no on-treatment failures. One patient relapsed after the end of treatment. The most common treatment-emergent adverse events were headache (8 %, 7/93) and fatigue (6 %, 6/93). There were no grade 3 or 4 adverse events, seven grade 3 laboratory abnormalities, and one premature discontinuation of study treatment (due to nonserious mouth ulceration). None of the three reported serious adverse events were related to treatment.
Conclusions
These data suggest that 12 weeks of ledipasvir/sofosbuvir is effective and well tolerated in treatment-naïve and treatment-experienced Korean patients with chronic genotype 1 HCV infection.

Similar content being viewed by others
References
Lim YS. Current status of liver disease in Korea: hepatitis C. Korean J Hepatol 2009;15(Suppl 6):S25–S28
Kim MN, Kim BK, Han KH. Hepatocellular carcinoma in patients with chronic hepatitis C virus infection in the Asia-Pacific region. J Gastroenterol 2013;48:681–688
Lavanchy D. Evolving epidemiology of hepatitis C virus. Clin Microbiol Infect 2011;17:107–115
Kim do Y, Kim IH, Jeong SH, et al. A nationwide seroepidemiology of hepatitis C virus infection in South Korea. Liver Int 2013;33:586–594
Oh DJ, Park YM, Seo YI, Lee JS, Lee JY. Prevalence of hepatitis C virus infections and distribution of hepatitis C virus genotypes among Korean blood donors. Ann Lab Med 2012;32:210–215
Cho EJ, Jeong SH, Han BH, Lee SU, Yun BC, Park ET. Hepatitis C virus (HCV) genotypes and the influence of HCV subtype 1b on the progression of chronic hepatitis C in Korea: a single center experience. Clin Mol Hepatol 2012;18:219–224
Korean Association for the Study of the Liver (KASL). KASL clinical practice guidelines: management of hepatitis C. Clin Mol Hepatol 2014;20:89–136
Park SY, Rim MY, Yo IK, et al. [Efficacy of peginterferon and ribavirin combination therapy of chronic hepatitis C: a pooled analysis]. Korean J Gastroenterol 2012;60:306–314
Conjeevaram HS, Fried MW, Jeffers LJ, Virahep-C Study Group, et al. Peginterferon and ribavirin treatment in African American and Caucasian American patients with hepatitis C genotype 1. Gastroenterology 2006;131:470–477
Muir AJ, Bornstein JD, Killenberg PG, Atlantic Coast Hepatitis Treatment Group. Peginterferon alfa-2b and ribavirin for the treatment of chronic hepatitis C in blacks and non-Hispanic whites. N Engl J Med 2004;350:2265–2271
Manns MP, McHutchison JG, Gordon SC, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet 2001;358:958–965
Hadziyannis SJ, Sette H Jr, Morgan TR, PEGASYS International Study Group, et al. Peginterferon-alpha2a and ribavirin combination therapy in chronic hepatitis C: a randomized study of treatment duration and ribavirin dose. Ann Intern Med 2004;140:346–355
Legrand-Abravanel F, Colson P, Leguillou-Guillemette H, et al. Influence of the HCV subtype on the virological response to pegylated interferon and ribavirin therapy. J Med Virol 2009;81:2029–2035
Jensen DM, Morgan TR, Marcellin P, et al. Early identification of HCV genotype 1 patients responding to 24 weeks peginterferon alpha-2a (40 kd)/ribavirin therapy. Hepatology 2006;43:954–960
Nicot F, Alric L, Barange K, et al. Influence of HCV genotype 1 subtypes on the virus response to PEG interferon alpha-2a plus ribavirin therapy. J Med Virol 2011;83:437–444.
Lyoo K, Song MJ, Hur W, et al. Polymorphism near the IL28B gene in Korean hepatitis C virus-infected patients treated with peg-interferon plus ribavirin. J Clin Virol 2011;52:363–366
Jung YK, Kim JH, Ahn SM, et al. Role of interleukin 28B-related gene polymorphisms in chronic hepatitis C and the response to antiviral therapy in Koreans. J Clin Gastroenterol 2013;47:644–650
Thomas DL, Thio CL, Martin MP, et al. Genetic variation in IL28B and spontaneous clearance of hepatitis C virus. Nature 2009;461:798–801
Kim SU, Song KJ, Chang HY, et al. Association between IL28B polymorphisms and spontaneous clearance of hepatitis B virus infection. PLoS ONE 2013;8:e69166
Fried MW, Shiffman ML, Reddy KR, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med 2002;347:975–982
Höroldt B, Haydon G, O’Donnell K, Dudley T, Nightingale P, Mutimer D. Results of combination treatment with pegylated interferon and ribavirin in cirrhotic patients with hepatitis C infection. Liver Int 2006;26:650–659.
Carrión JA, Martínez-Bauer E, Crespo G, et al. Antiviral therapy increases the risk of bacterial infections in HCV-infected cirrhotic patients awaiting liver transplantation: a retrospective study. J Hepatol 2009;50:719–728
Huang CF, Yang JF, Dai CY, et al. Efficacy and safety of pegylated interferon combined with ribavirin for the treatment of older patients with chronic hepatitis C. J Infect Dis 2010;201:751–759
Thabut D, Le Calvez S, Thibault V, et al. Hepatitis C in 6865 patients 65 yr or older: a severe and neglected curable disease? Am J Gastroenterol 2006;101:1260–1267
Nudo CG, Wong P, Hilzenrat N, Deschênes M. Elderly patients are at greater risk of cytopenia during antiviral therapy for hepatitis C. Can J Gastroenterol 2006;20:589–592
Marcus EL, Tur-Kaspa R. Chronic hepatitis C virus infection in older adults. Clin Infect Dis 2005;41:1606–1612
Gilead Sciences, Inc. HARVONI (ledipasvir and sofosbuvir) prescribing information. Foster City: Gilead Sciences, Inc.; 2014.
Gilead Sciences International Ltd. HARVONI (ledipasvir/sofosbuvir) summary of product characteristics. Cambridge: Gilead Sciences International Ltd.; 2014
Afdhal N, Zeuzem S, Kwo P, et al. ION-1 Investigators. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med 2014;370:1889–1898
Kowdley KV, Gordon SC, Reddy KR, et al. ION-3 Investigators. Ledipasvir and sofosbuvir for 8 or 12 weeks for chronic HCV without cirrhosis. N Engl J Med 2014;370:1879–1888
Afdhal N, Reddy KR, Nelson DR, et al. ION-2 Investigators. Ledipasvir and sofosbuvir for previously treated HCV genotype 1 infection. N Engl J Med 2014;370:1483–1493
Mizokami M, Yokosuka O, Takehara T, et al. Ledipasvir and sofosbuvir fixed-dose combination with and without ribavirin for 12 weeks in treatment-naive and previously treated Japanese patients with genotype 1 hepatitis C: an open-label, randomised, phase 3 trial. Lancet Infect Dis 2015;15:645–653
Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron 1976;16:31–41
Clopper CJ, Pearson ES. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika 1934;26:404–413
García-Samaniego J, Romero M, Granados R, et al. Factors associated with early virological response to peginterferon-α-2a/ribavirin in chronic hepatitis C. World J Gastroenterol 2013;19:1943–1952
Bressler BL, Guindi M, Tomlinson G, Heathcote J. High body mass index is an independent risk factor for nonresponse to antiviral treatment in chronic hepatitis C. Hepatology 2003;38:639–644
Acknowledgements
This study was funded in full by Gilead Sciences, Inc. The writing and preparation of this paper were funded by Gilead Sciences, Inc. The initial data analyses were undertaken by the listed authors. Writing support was provided by Tiffany DeSimone, PhD, of BlueMomentum, an Ashfield Company, part of UDG Healthcare plc, and funded by Gilead Sciences, Inc.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Young-Suk Lim has served as an advisor for Bayer Healthcare, Bristol-Myers Squibb, and Gilead Sciences, and has received research funding from Bayer Healthcare, Bristol-Myers Squibb, Gilead Sciences, and Novartis. Sang Hoon Ahn has served as an advisor and lecturer for Bristol-Myers Squibb, Gilead Sciences, F. Hoffmann-La Roche, Merck, Janssen, AbbVie, Novartis, Boeringher Ingelheim, and AVIVAX, and has received unrestricted grants from Bristol-Myers Squibb, Gilead Sciences, and F. Hoffmann-La Roche for investigator-initiated trials. Seung Woon Paik has received research funding from Bristol-Myers Squibb, Gilead Sciences, F. Hoffmann-La Roche, Merck, Janssen, and AbbVie. Hyung Joon Yim has received research funds from Handok Pharmacuetical Company and Gilead Sciences. Yoon Jun Kim has served as a speaker and advisory board member for Gilead Sciences, Bayer Healthcare, AbbVie, Novartis, and F. Hoffman-La Roche and has also received research funding from Gilead Sciences, Bristol-Myers Squibb, and F. Hoffman-La Roche. Kwan-Soo Byun has served as an advisory board member for Gilead Sciences and has received research funding from Gilead Sciences, Bristol-Myers Squibb, and Medigen Biotechnology Corporation. Jenny C. Yang, Hongmei Mo, Kimberly L. Garrison, Bing Gao, Steven J. Knox, and Phillip S. Pang are employees of and own stocks and shares in Gilead Sciences, Inc. Kwan Sik Lee, Youn-Jae Lee, Sook-Hyang Jeong, Ju-Hyun Kim, Seung Kew Yoon, Won Young Tak, Sang-Young Han, Young Seok Kim, Jeong Heo, and Kwang-Hyub Han have nothing to declare.
Studies with human subjects
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008. Written informed consent was obtained from all patients included in the study. The protocol was approved by the ethics committees/institutional review boards of participating centers and conformed to Good Clinical Practice guidelines and Declaration of Helsinki principles. This article does not contain any studies with animal subjects.
Additional information
Y.-S. Lim, S.H. Ahn contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lim, YS., Ahn, S.H., Lee, K.S. et al. A phase IIIb study of ledipasvir/sofosbuvir fixed-dose combination tablet in treatment-naïve and treatment-experienced Korean patients chronically infected with genotype 1 hepatitis C virus. Hepatol Int 10, 947–955 (2016). https://doi.org/10.1007/s12072-016-9726-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12072-016-9726-5