Abstract
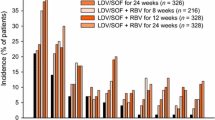
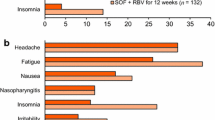
A single-tablet regimen of ledipasvir and sofosbuvir (ledipasvir/sofosbuvir; Harvoni®) was recently approved in the EU. The phase 3 ION trials included treatment-naïve (ION-1 and -3) or treatment-experienced (ION-2) patients with chronic hepatitis C virus (HCV) genotype 1 infection; ≈20 % of patients in ION-1 and -2 had cirrhosis, whereas none of the patients in ION-3 had cirrhosis. In ION-1, a 12-week regimen of ledipasvir/sofosbuvir achieved high rates of sustained virological response 12 weeks’ post-treatment (SVR12) in treatment-naïve patients, with no additional benefit conferred by the addition of ribavirin or extending the treatment duration to 24 weeks. An 8-week regimen also achieved high SVR12 rates in patients with a baseline HCV RNA level of <6 million IU/mL in ION-3. High SVR12 rates were seen in treatment-experienced patients who received ledipasvir/sofosbuvir for 12 or 24 weeks in ION-2. Data also support the use of ledipasvir/sofosbuvir in chronic HCV genotype 4 infection, in HCV and HIV co-infection and, in combination with ribavirin, in patients with chronic HCV genotype 1 or 4 infection who have decompensated cirrhosis or are liver transplant recipients and in chronic HCV genotype 3 infection. Oral ledipasvir/sofosbuvir was generally well tolerated.
Similar content being viewed by others
References
European Association for the Study of the Liver. EASL recommendations on treatment of hepatitis C 2015. J Hepatol. 2015;63(1):199–236.
American Association for the Study of Liver Diseases and Infectious Diseases Society of America. Recommendations for testing, managing, and treating hepatitis C (December 19). 2014. http://www.hcvguidelines.org/full-report-view. Accessed 29 Apr 2015.
Harvoni (ledipasvir/sofosbuvir) film-coated tablets: EU summary of product characteristics. London: European Medicines Agency; 2015.
Hebner C, Lee Y-J, Han B, et al. In vitro pan-genotypic and combination activity of sofosbuvir (GS-7977) in stable replicon cell lines [abstract no. 1875]. Hepatology. 2012;56(4 Suppl):1066A.
Afdhal N, Zeuzem S, Kwo P, et al. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med. 2014;370(20):1889–98.
Afdhal N, Reddy KR, Nelson DR, et al. Ledipasvir and sofosbuvir for previously treated HCV genotype 1 infection. N Engl J Med. 2014;370(16):1483–93.
Kowdley KV, Gordon SC, Reddy KR, et al. Ledipasvir and sofosbuvir for 8 or 12 weeks for chronic HCV without cirrhosis. N Engl J Med. 2014;370(20):1879–88.
EMA recommends avoidance of certain hepatitis C medicines and amiodarone together. London: European Medicines Agency; 2015.
Bernstein DE, Mangia A, Brau N, et al. Concordance between SVR4, SVR12 and SVR24 in genotype 1 HCV-infected patients who received all oral fixed-dose combination ledipasvir/sofosbuvir with or without ribavirin in phase 3 clinical trials [abstract no. 1947 plus slide presentation]. Hepatology. 2014;60(4 Suppl):1142A–3A.
Jacobson IM, Kwo PY, Kowdley KV, et al. Virologic response rates to all oral fixed-dose combination ledipasvir/sofosbuvir regimens are similar in patients with and without traditional negative predictive factors in phase 3 clinical trials [abstract no. 1945 plus poster]. Hepatology. 2014;60(4 Suppl):1141A–2A.
Gordon SC, Fried MW, Kwo PY, et al. No differences in the efficacy of fixed-dose combination ledipasvir/sofosbuvir in patients according to fibrosis stage determined by liver biopsy or laboratory biomarker in phase 3 clinical trials [abstract no. 1958 plus poster]. Hepatology. 2014;60(4 Suppl):1149A–50A.
Naggie S, Cooper C, Saag MS, et al. Ledipasvir/sofosbuvir for 12 weeks in patients coinfected with HCV and HIV-1 [abstract no. 152LB]. In: 2015 Conference on Retroviruses and Opportunistic Infections. 2015.
Reddy KR, Everson GT, Flamm SL, et al. Ledipasvir/sofosbuvir with ribavirin for the treatment of HCV in patients with post-transplant recurrence: preliminary results of a prospective, multicenter study [abstract no. 8 plus slide presentation]. Hepatology. 2014;60(4 Suppl):200A–1A.
Flamm SL, Everson GT, Charlton M, et al. Ledipasvir/sofosbuvir with ribavirin for the treatment of HCV in patients with decompensated cirrhosis: preliminary results of a prospective multicenter study [abstract no. 239 plus slide presentation]. Hepatology. 2014;60(4 Suppl):320A–1A.
Gane E, Hyland RH, Pang P, et al. Sofosbuvir/ledipasvir fixed dose combination is safe and effective in HCV infected populations including decompensated patients and patients with prior sofosbuvir treatment experience [abstract no. 238 plus slide presentation]. Gastroenterology. 2014;146(5 Suppl 1):S904–5.
Gane EJ, Hyland RH, An D, et al. High efficacy of LDV/SOF regimens for 12 weeks for patients with HCV genotype 3 or 6 infection [abstract no. LB-11 plus poster]. Hepatology. 2014;60(1 Suppl):22.
Kapoor R, Kohil A, Sidharthan S, et al. All oral treatment for genotype 4 chronic hepatitis C infection with sofosbuvir and ledipasvir: interim results from the NIAID SYNERGY trial [abstract no. 240 plus slide presentation]. Hepatology. 2014;60(4 Suppl):321A.
Manns M, Forns X, Samuel D, et al. Ledipasvir/sofosbuvir with ribavirin is safe and efficacious in decompensated and post liver transplantation patients with HCV infection: preliminary results of the prospective SOLAR 2 trial [abstract no. G02]. J Hepatol. 2015;62(Suppl):S187–8.
Abergel A, Loustaud-Ratti V, Metivier S, et al. Ledipasvir/sofosbuvir treatment results in high SVR rates in patients with chronic genotype 4 and 5 HCV infection [abstract no. O056]. J Hepatol. 2015;62(Suppl):S219–20.
Younossi ZM, Stepanova M, Afdhal N, et al. Improvement of health-related quality of life and work productivity in chronic hepatitis C patients with early and advanced fibrosis treated with ledipasvir and sofosbuvir. J Hepatol. 2015;63(2):337–45.
Younossi ZM, Stepanova M, Marcellin P, et al. Treatment with ledipasvir and sofosbuvir improves patient-reported outcomes: results from the ION-1, 2 and 3 clinical trials. Hepatology. 2015;61(6):1798–808.
Mizokami M, Yokosuka O, Takehara T, et al. Ledipasvir and sofosbuvir fixed-dose combination with and without ribavirin for 12 weeks in treatment-naive and previously treated Japanese patients with genotype 1 hepatitis C: an open-label, randomised, phase 3 trial. Lancet Infect Dis. 2015;15(6):645–53.
Bourlière M, Bronowicki JP, de Ledinghen V, et al. Ledipasvir-sofosbuvir with or without ribavirin to treat patients with HCV genotype 1 infection and cirrhosis non-responsive to previous protease-inhibitor therapy: a randomised, double-blind, phase 2 trial (SIRIUS). Lancet Infect Dis. 2015;15(4):397–404.
Reddy KR, Bourliere M, Sulkowski M, et al. Ledipasvir and sofosbuvir in patients with genotype 1 HCV and compensated cirrhosis: an integrated safety and efficacy analysis. Hepatology. 2015;62(1):79–86.
Osinusi A, Townsend K, Kohli A, et al. Virologic response following combined ledipasvir and sofosbuvir administration in patients with HCV genotype 1 and HIV co-infection. JAMA. 2015;313(12):1232–9.
Alqahtani SA, Afdhal N, Zeuzem S, et al. Safety and tolerability of ledipasvir/sofosbuvir with and without ribavirin in patients with chronic hepatitis C virus genotype 1 infection: analysis of phase III ION trials. Hepatology. 2015;62(1):25–30.
Wyles D, Pockros P, Morelli G, et al. Ledipasvir-sofosbuvir plus ribavirin for patients with genotype 1 hepatitis C virus previously treated in clinical trials of sofosbuvir regimens. Hepatology. 2015;61(6):1793–7.
Keating GM. Ledipasvir/sofosbuvir: a review of its use in chronic hepatitis C. Drugs. 2015;75(6):675–85.
Acknowledgments
The review was updated from Drugs 2015;75(6):675–85 [28] and was reviewed by: S. Saluja, Saran Ashram Hospital, Dayalbagh, Agra, India; R. B. Shah, Department of Pharmacology, GMERS Medical College and Hospital, Gandhinagar, Gujarat, India. During the peer review process, the manufacturer of the agent under review was offered an opportunity to comment on the manuscript. Changes resulting from comments received were made by the authors on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Conflict of interest
Gillian M. Keating is a salaried employee of Adis/Springer, is responsible for the article content and declares no relevant conflicts of interest.
Rights and permissions
About this article
Cite this article
Keating, G.M. Ledipasvir/sofosbuvir in chronic hepatitis C: a guide to its use in the EU. Drugs Ther Perspect 31, 289–295 (2015). https://doi.org/10.1007/s40267-015-0232-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40267-015-0232-y