Abstract
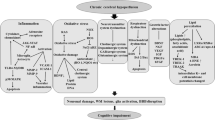
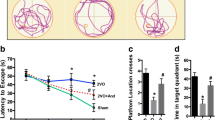
Iron is a highly reactive free radical catalyst that has been shown to exacerbate oxidative stress and cell death in many neurodegenerative diseases. In this study, we produced a rat model of chronic cerebral hypoperfusion (CCH) by permanent bilateral carotid artery occlusion to investigate markers of iron and oxidative stress associated with it. We found CCH led to significant spatial memory impairment in the Morris water maze at 4 months after bilateral ligation. Iron deposition was observed in both the hippocampal CA1 area and cerebral cortex, and was correlated with localized neuronal death and increased lipid peroxidation. Western blotting revealed that the expression levels of ferritin heavy chain and the transferrin receptor were significantly elevated in hippocampus and cortex after CCH, whereas expression of iron regulatory protein 1 was significantly lower than in sham-treated rats. We conclude that localized neurodegeneration and concomitant cognitive impairments following CCH may result, at least in part, from local disruption of neuronal iron metabolism.




Similar content being viewed by others
References
Iadecola C (2004) Neurovascular regulation in the normal brain and in Alzheimer's disease. Nat Rev Neurosci 5:347–360
Zlokovic BV (2005) Neurovascular mechanisms of Alzheimer's neurodegeneration. Trends Neurosci 28:202–208
Ruitenberg A, den Heijer T, Bakker SL, van Swieten JC, Koudstaal PJ, Hofman A, Breteler MM (2005) Cerebral hypoperfusion and clinical onset of dementia: the Rotterdam Study. Ann Neurol 57:789–794
Lee JS, Im DS, An YS, Hong JM, Gwag BJ, Joo IS (2011) Chronic cerebral hypoperfusion in a mouse model of Alzheimer's disease: an additional contributing factor of cognitive impairment. Neurosci Lett 489:84–88
Farkas E, Luiten PG, Bari F (2007) Permanent, bilateral common carotid artery occlusion in the rat: a model for chronic cerebral hypoperfusion- related neurodegenerative diseases. Brain Res Rev 54:162–180
Götz ME, Künig G, Riederer P, Youdim MBH (1994) Oxidative stress: free radical production in neural degeneration. Pharmacol Ther 63:37–122
Yang L, Zhang B, Yin L, Cai B, Shan H, Zhang L, Lu Y, Bi Z (2011) Tanshinone IIA prevented brain iron dyshomeostasis in cerebral ischemic rats. Cell Physiol Biochem 27:23–30
Ni J, Ohta H, Matsumoto K, Watanabe H (1994) Progressive cognitive impairment following chronic cerebral hypoperfusion induced by permanent occlusion of bilateral carotid arteries in rats. Brain Res 653:231–236
Morris R (1984) Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods 11:47–60
Paxinos G, Watson C (2005) The rat brain in stereotaxic coordinates, 6th edn. Academic, Sydney
Dai X, Chen L, Sokabe M (2007) Neurosteroid estradiol rescues ischemia-induced deficit in the long-term potentiation of rat hippocampal CA1 neurons. Neuropharmacol 52:1124–1138
Nguyen-Legros J, Bizot J, Bolesse M, Pulicani JP (1980) “Diaminobenzidine black” as a new histochemical demonstration of exogenous iron. Histochemistry 66:239–244
Zecca L, Youdim MB, Riederer P, Connor JR, Crichton RR (2004) Iron, brain ageing and neurodegenerative disorders. Nat Rev Neurosci 5:863–873
Bishop G, Robinson S (2001) Quantitative analysis of cell death and ferritin expression in response to cortical iron, implications for hypoxia-ischemia and stroke. Brain Res 907:175–187
Klausner RD, Rouault TA, Harford JB (1993) Regulating the fate of mRNA: the control of cellular iron metabolism. Cell 72:19–28
Bonkovsky HL, Ponka P, Bacon BR, Drysdale J, Grace ND, Tavill AS (1996) An update on iron metabolism: summary of the Fifth International Conference on Disorders of Iron Metabolism. Hepatology 24:718–729
Hentze MW, Kuhn LC (1996) Molecular control of vertebrate iron metabolism: mRNA-based regulatory circuits operated by iron, nitric oxide, and oxidative stress. Proc Natl Acad Sci U S A 93:8175–8182
Qian ZM (2002) Nitric oxide and changes of iron metabolism in exercise. Biol Rev 77:529–536
Chasteen ND (1998) Ferritin. Uptake, storage, and release of iron. Met Ions Biol Syst 35:479–514
Rucker P, Torti FM, Torti SV (1996) Role of H and L subunits in mouse ferritin. J Biol Chem 271:33352–33357
Friedman A, Arosio P, Finazzi D, Koziorowski D, Galazka-Friedman J (2011) Ferritin as an important player in neurodegeneration. Parkinsonism Relat Disord 17:423–430
Harrison PM, Arosio P (1996) The ferritins: molecular properties, iron storage function and cellular regulation. Biochim Biophys Acta 1275:161–203
Lin F, Girotti AW (1997) Elevated ferritin production, iron containment, and oxidant resistance in hemin-treated leukemia cells. Arch Biochem Biophys 346:131–141
Garner B, Roberg K, Brunk UT (1998) Endogenous ferritin protects cells with iron-laden lysosomes against oxidative stress. Free Radic Res 29:103–114
Oberle S, Polte T, Abate A, Podhaisky HP, Schroder H (1998) Aspirin increases ferritin synthesis in endothelial cells: a novel antioxidant pathway. Circ Res 82:1016–1020
Mandel S, Grunblatt E, Riederer P (2011) Iron in brain function and neurodegenerative disorders. Editorial J Neural Transm 118:299–300
Pinero DJ, Hu J, Cook BM, Scaduto RC Jr, Connor JR (2000) Interleukin-1beta increases binding of the iron regulatory protein and the synthesis of ferritin by increasing the labile iron pool. Biochim Biophys Acta 1497:279–288
LaVaute T, Smith S, Cooperman S, Iwai K, Land W, Meyron-Holtz E, Drake SK, Miller G, Abu-Asab M, Tsokos M, Tsokos M, Switzer R 3rd, Grinberg A, Love P, Tresser N, Rouault TA (2001) Targeted deletion of the gene encoding iron regulatory protein-2 causes misregulation of iron metabolism and neurodegenerative disease in mice. Nat Genet 27:209–214
Smith SR, Cooperman S, Lavaute T, Tresser N, Ghosh M, Meyron-Holtz E, Land W, Ollivierre H, Jortner B, Switzer R 3rd, Messing A, Rouault TA (2004) Severity of neurodegeneration correlates with compromise of iron metabolism in mice with iron regulatory protein deficiencies. Ann N Y Acad Sci 1012:65–83
Cooperman SS, Meyron-Holtz EG, Olivierre-Wilson H, Ghosh MC, McConnell JP, Rouault TA (2005) Microcytic anemia, erythropoietic protoporphyria, and neurodegeneration in mice with targeted deletion of iron-regulatory protein 2. Blood 106:1084–1091
Kato J, Fujikawa K, Kanda M, Fukuda N, Sasaki K, Takayama T, Kobune M, Takada K, Takimoto R, Hamada H, Ikeda T, Niitsu Y (2001) A mutation, in the iron-responsive element of H ferritin mRNA, causing autosomal dominant iron overload. Am J Hum Genet 69:191–197
Li L, Li YW, Zhao JY, Liu YZ, Holscher C (2009) Quantitative analysis of ironconcentration and expression of ferroportin 1 in the cortex and hippocampus of rats induced by cerebral ischemia. J Clin Neurosci 11:1466–1472
Acknowledgments
This study was funded by Shanghai Municipal Health Bureau grant KPB-WSJ1004.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, Y., He, Y., Guan, Q. et al. Disrupted Iron Metabolism and Ensuing Oxidative Stress may Mediate Cognitive Dysfunction Induced by Chronic Cerebral Hypoperfusion. Biol Trace Elem Res 150, 242–248 (2012). https://doi.org/10.1007/s12011-012-9455-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-012-9455-0